Equilibria 1.6
1/11
Earn XP
Description and Tags
le chateliers principle and factors that affect the equilibrium position
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
what type of reaction can reach dynamic equilibrium?
Reversible reactions
Defintion for Le Chanteliers principle
When a system is subject to change the system will alter to lessen the effect of that change
How does increasing the temperature effect a reversible reaction?
Increasing the temperature favors the endothermic reaction. Therefore increasing the yield of endothermic products
How does decreasing the temperature effect a reversible reaction?
Decreasing the temperature favors the exothermic reaction. therefore increasing the yield of exothermic products.
How does increasing the pressure effect a reversible reaction?
increasing the pressure favors the side with less moles. as it will help decrease the pressure. this will increase the yield of products on this side.
How does decreasing the pressure effect a reversible reaction?
decreasing the pressure favors the side with more moles. as it will help increase the pressure. This will increase the yield of products on this side.
How does increasing the concentration of reactants effect a reversible reaction?
Increasing the conc. of reactants will produce more products as more molecules are available to react.
therefore increase the yield of products.
How does adding a catalyst affect a reversible reaction?
They do not affect the equilibrium position.
But they do affect the forward and backwards reaction equally.
and equilibrium is reached faster.
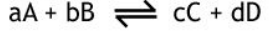
what is the formula for Kc?

What do the square brackets each represent>
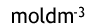
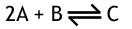
What is the unit for Kc in this reversible reaction?
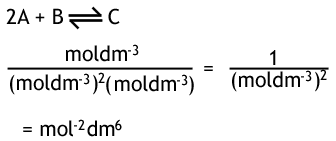
What is the only thing Kc can be affects by?
temperature