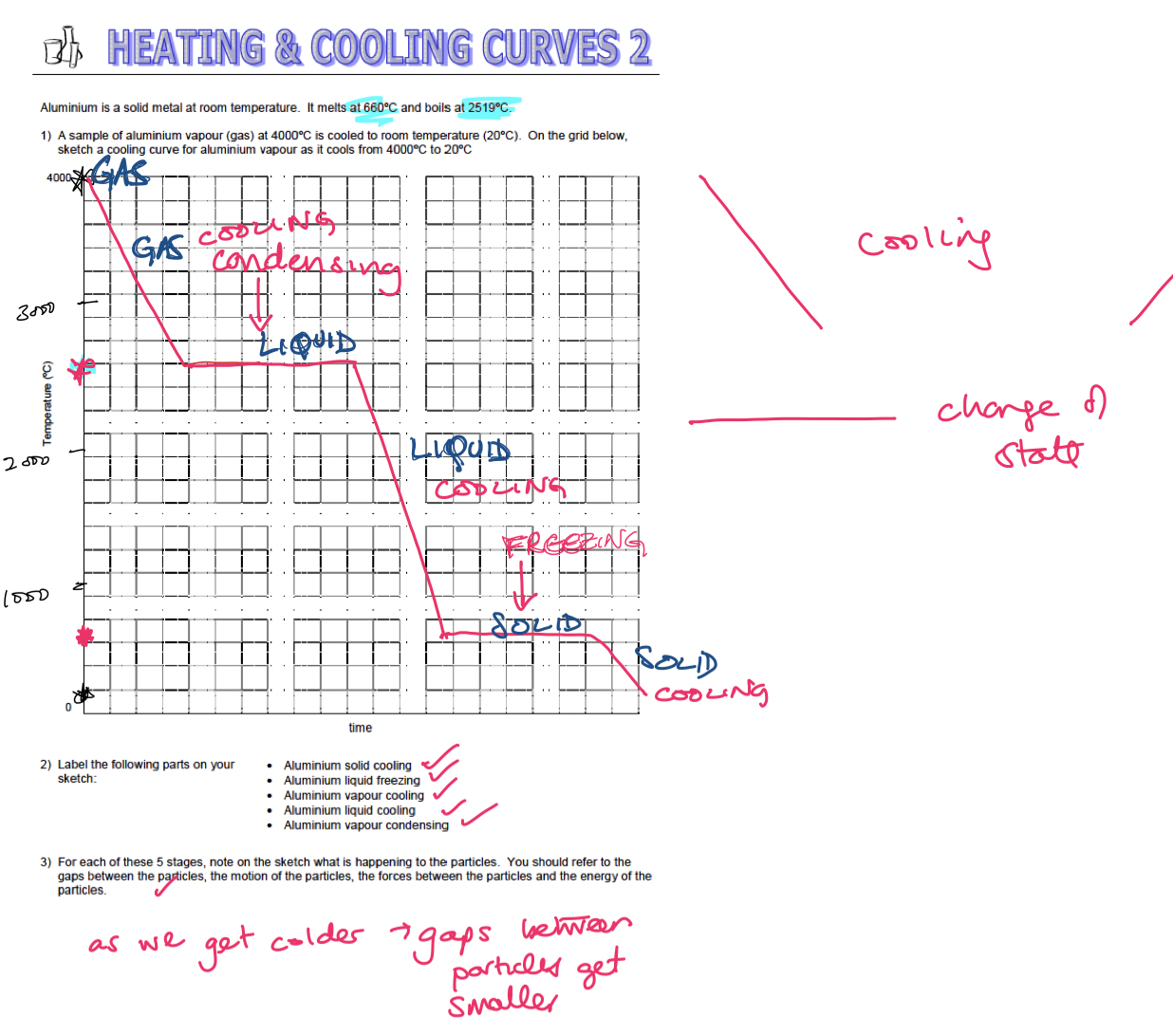
1.1.1 - Heating and Cooling Curves
0.0(0)
Card Sorting
1/17
There's no tags or description
Looks like no tags are added yet.
Last updated 4:04 PM on 5/17/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
1
New cards
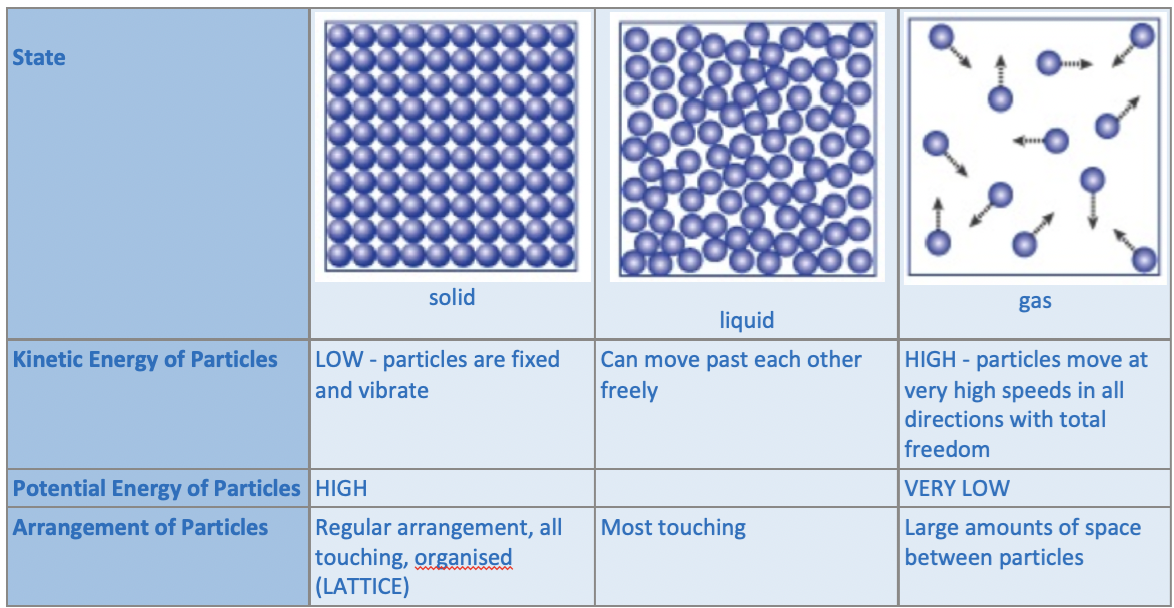
Kinetic Molecular Theory (Particle Theory)
\* Inserted Image
2
New cards
State: Solid
Kinetic Energy of Particles:
Kinetic Energy of Particles:
LOW → particles don’t move as much as they are in a fixed position and vibrate
3
New cards
State: Solid
Potential Energy of Particles:
Potential Energy of Particles:
HIGH
4
New cards
State: Solid
Arrangement of Particles:
Arrangement of Particles:
Regular arrangement, all particles are touching, organised (lattice)
5
New cards
State: Liquid
Kinetic Energy of Particles:
Kinetic Energy of Particles:
Can move past each other freely
6
New cards
State: Liquid
Potential Energy of Particles:
Potential Energy of Particles:
VARIES → depending on the intermolecular forces between the particles
7
New cards
State: Liquid
Arrangement of Particles:
Arrangement of Particles:
Most touching
8
New cards
State: Gas
Kinetic Energy of Particles:
Kinetic Energy of Particles:
HIGH - particles move at very high speeds in all directions with total freedom
9
New cards
State: Gas
Potential Energy of Particles:
Potential Energy of Particles:
VERY LOW
10
New cards
State: Gas
Arrangement of Particles:
Arrangement of Particles:
Large amounts of space between particles
11
New cards
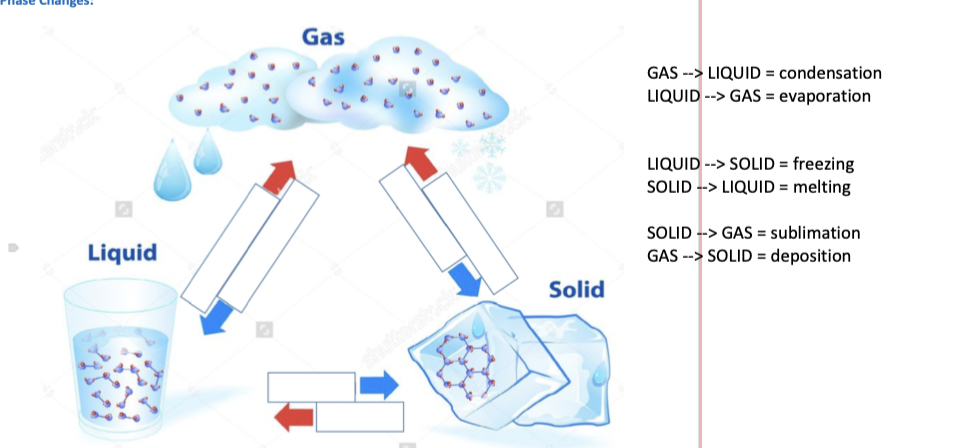
Phase Changes
\*Inserted Image
12
New cards
Gas to Liquid
CONDENSATION
13
New cards
Liquid to Gas
EVAPORATION
14
New cards
Liquid to Solid
FREEZING
15
New cards
Solid to Liquid
MELTING
16
New cards
Solid to Gas
SUBLIMATION
17
New cards
Gas to Solid
DEPOSITION
18
New cards
Heating and Cooling Curves:
\*inserted image