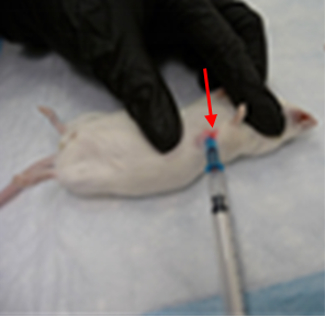
Lab Animal Restraint & Venipuncture
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
11 Terms
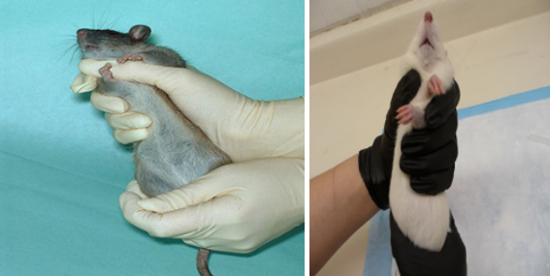
Shoulder/Two-Finger Hold
Grasp the tail base with your dominant hand.
With the other hand, slide your thumb and index finger up under shoulders, forcing them upward until the forelegs are crisscrossed. Now place your dominant hand around the hips to secure their hindlimbs, always supporting its back.
Gently stretch the animal to maintain upward lift of the shoulders.
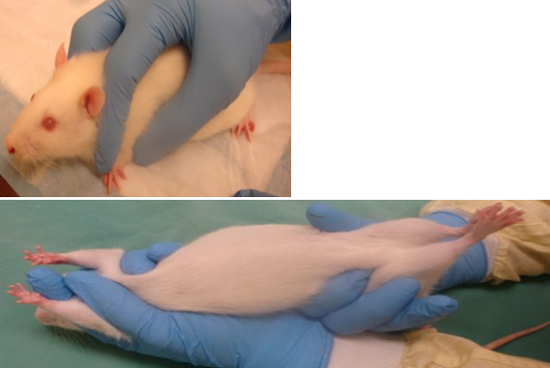
“V” Hold
Grasp the tail base with your dominant hand, slide the other hand forward, placing the thumb under the leg and shoulder on one side, and the index finger on top of the shoulder and alongside the neck on the other side.
Place your middle finger on the other side of the head to form a “V”. Now place your dominant hand around the hips to secure their hindlimbs, always supporting its back.
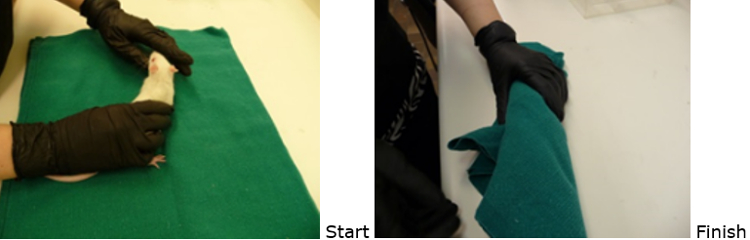
Burrito
Place the rat on a towel.
Drape one corner of the towel over the rat’s face while still holding the rat’s hind end to prevent escape.
Fold each side over to form a “burrito”.
Gently roll and tuck the rat in the towel securely. Ensure the rat is right side up when complete.
Injections
The three main injection sites in mice and rats are subcutaneous, intraperitoneal, and intravenous.
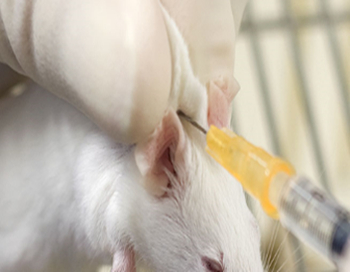
Subcutaneous (SQ)
The most common sites are along the animal’s back. Lift the skin over the shoulder or lumbar area to form a tent.
Insert the needle bevel up at the tent base, holding the needle parallel to the animal’s body while injecting.
Withdraw the needle and press the skin to seal the needle’s exit hole and to prevent any fluid leakage.

Intraperitoneal (IP)
First, know your anatomy. To locate the point of entry for the needle, draw an imaginary line across the abdomen just above the knees and down the midline of the animal. Their right side holds the small intestines, and the left side holds the cecum. Inserting the needle too far caudally or laterally from the insertion point would risk making an injection into the rear leg, which may injure muscle tissue.
Tilt the animal downward on an approximate 25–30° angle to allow cranial movement of the organs.
Insert the needle along this line on either side of the animal, and close to the midline (where the dots are located in the photo). Mice require one person for restraint and injection. Rats require two people for an IP injection—one to hold and the other to inject.

Intravenous (IV)
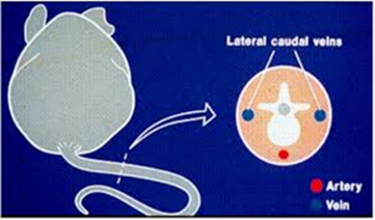
Tail veins are the most common venous site. There are two veins on either side of the tail (in blue). The arteries are on the top and bottom of the tail (in red) and should be avoided.
Restrain the animal by using a restraint device or anesthesia; hand restraint is not recommended.
Using your non-dominant hand, hold the tail using your first two fingers at the tail base and the rest of your fingers down at the tip. Pull slightly to straighten and support the tail. Locate the right or left lateral tail vein by slightly turning it to either side so the vein is on top of the tail.
Prepare the position by slightly bending the distal portion of the tail down to create a slight angle to have the needle parallel to the vein, always keeping the tail straight and taut.
Insert the needle tip bevel up and anchor by holding the tail and hub with your non-dominant thumb and forefinger before injecting. Any white blebbing of the surrounding tail skin means the fluid has gone perivascular. If this occurs, remove and discard the needle to attempt again, only moving up the tail to the animal’s rump.
Blood Collection
The three main intravenous collection sites in mice and rats are the tail veins, saphenous veins, and the heart.
The blood volume of a mouse is approximately 2 ml. The blood volume of a rat is approximately 50 ml/kg. Removing greater quantities of blood (exceeding 10% of total blood volume) can produce hypovolemic shock.

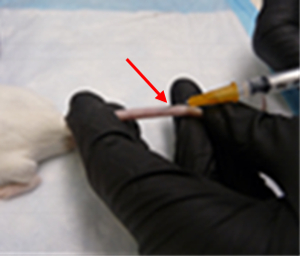
Tail Veins
Locate the right or left lateral tail vein by slightly turning it to either side so the vein is on top of the tail.
Using your non-dominant hand, hold the tail using your first two fingers at the tail base and the rest of your fingers down at the tip. Pull slightly to straighten and support the tail. Prepare the position by slightly bending the distal portion of the tail down to create a slight angle to have the needle parallel to the vein, always keeping the tail straight and taut.
Two effective approaches depend on volume required or comfort for the RVT. Below describes the use of a needle and syringe or a needle and collection tube.
Insert the needle tip bevel up and anchor by holding the tail and hub with non-dominant thumb and forefinger before proceeding. Aspirate the plunger slowly using a 0.5–1-ml syringe to obtain the sample, being careful not to collapse the vein. Remove the needle and apply pressure until the bleeding has stopped.
If using a hematocrit or Microvette tube, insert the needle tip bevel up to puncture the vein in the distal quarter to half of the tail at a 90° angle. Slowly withdraw the needle so a bleb of blood forms at the puncture site. Collect the sample with a tube, use gravity and angle downward. If blood slows or stops, gently wipe area with gauze to disturb the clot formation. Apply pressure until the bleeding has stopped.

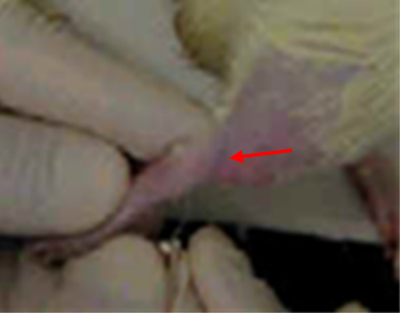
Saphenous Veins
The saphenous veins are large, subcutaneous, superficial veins of the hindlimbs.
Two effective approaches depend on comfort for the RVT. This is either using the medial (inner) or lateral (outer) vein. Two people may be required to achieve this procedure in rats. Always apply pressure until the bleeding has stopped.
After appropriate restraint, apply a silicone-based gel (e.g., Vaseline) on the skin to prevent blood from mixing with fur. Shaving or plucking is not required.
For the medial venous site, gently extend the outer hindlimb (closest to your baby finger) with your dominant hand and position your restraint hand over the vein with gentle pressure using one to two fingers over the inguinal area; this prevents the animal from pulling back its leg.
For the lateral venous site, it is recommended to restrain using the “burrito” method if non-anesthetized. Extend the hindlimb out of the towel. Hold off the vein with gentle pressure using your fingers of the restraint hand over the inguinal area; this prevents the animal from pulling back its leg.
Using a needle bevel up, puncture the vein at a 45 to 90° angle until the needle tip is just through the skin (the vessel is quite shallow). A bleb of blood should appear after removing the needle. Lighten up pressure over inguinal area to promote blood flow.
Collect blood sample with a Microvette or capillary tube by placing tip close to the bleb using gravity and angle downward. If blood slows or stops, gently wipe area with gauze to disturb the clot formation or softly pump the limb back and forth to increase outflow. Apply pressure until the bleeding has stopped.
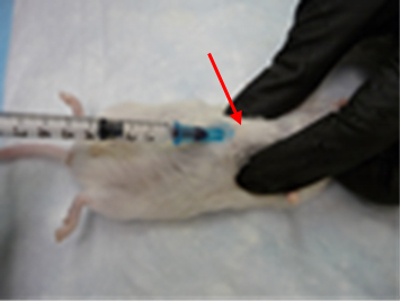
Heart-Intercardiac (IC)
This must be carried out on an anesthetized animal only. As a terminal procedure, this method is used to acquire the maximum volume of blood. Cardiac collection yields an average of 1–1.5 ml from mice and 10–15 ml from rats.
Two effective approaches depend on comfort for the RVT. This is either positioning the animal dorsally (on its back) or laterally (on its side).
Using the dorsal method, palpate the xiphoid cartilage and feel for the notch at the base of the sternum. Insert the needle bevel up at a 30–60° angle at the notch, and advance the needle toward the head.
Using the lateral method, locate the landmark on the left side of the animal’s chest at the base of the elbow and palpate to feel where the heartbeat is strongest. Insert the needle bevel up between two ribs into the heart.
Blood will be visible in the hub of the needle when the heart is punctured. Slowly aspirate the plunger to collect the sample. When finished, euthanize the animal immediately.