11-Alcohols, Ethers, and Epoxides
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
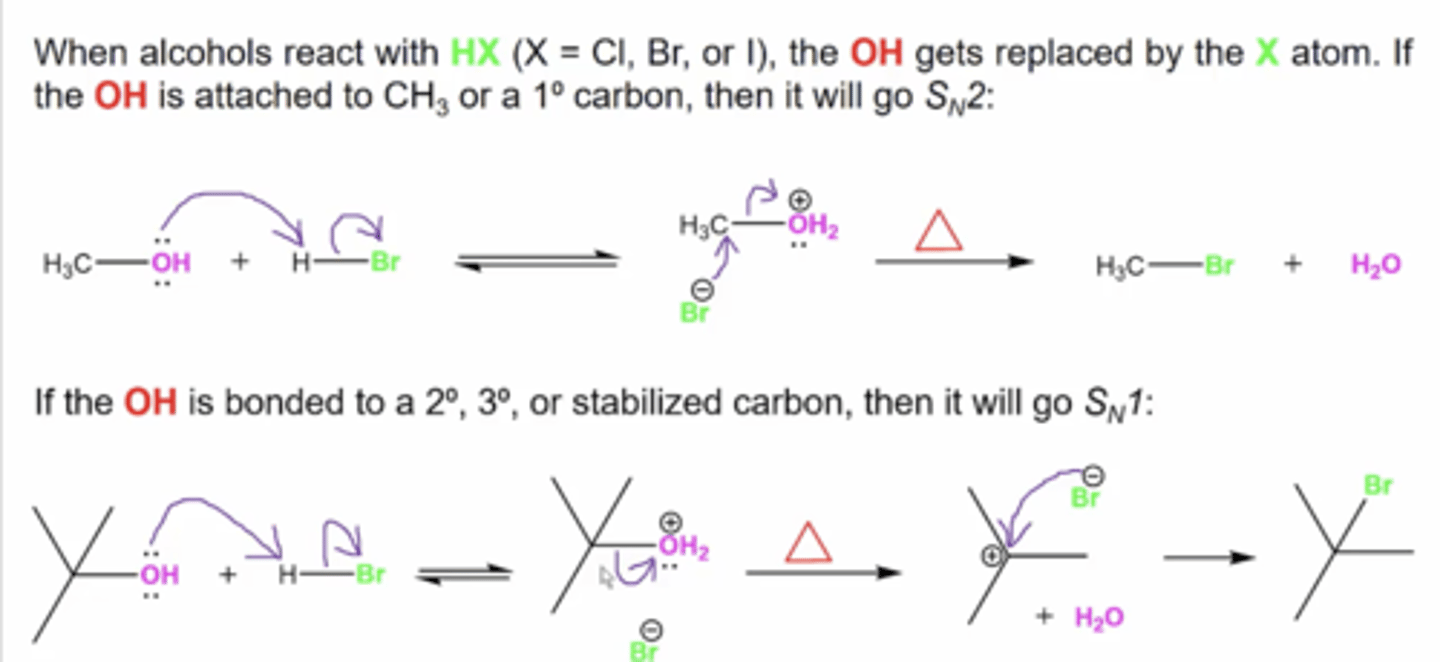
what happens when alcohols react with HX? (X=Cl, Br, or I)
OH gets replaced by the X atom.
-if the OH is attached to CH3 or a primary carbon, then it will go Sn2
-if the OH is attached to a secondary, tertiary, or stabilized carbon, it will go Sn1
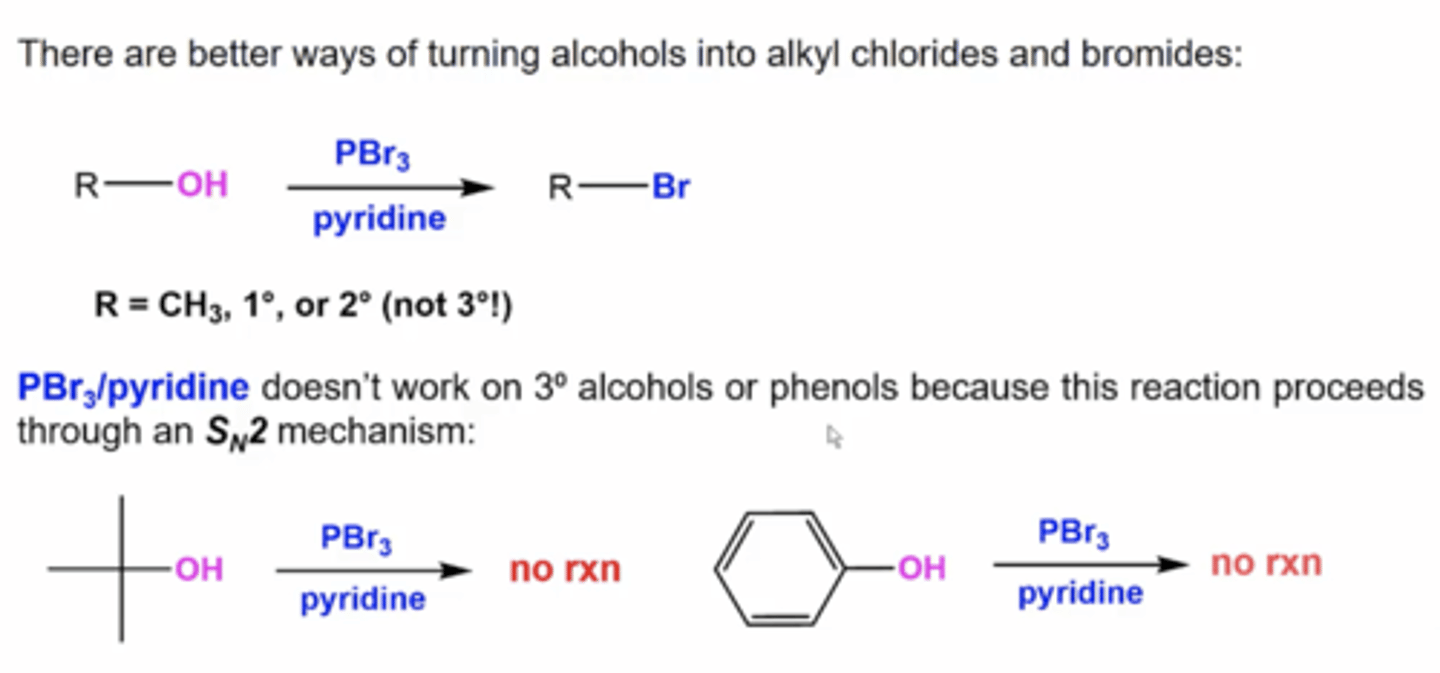
what happens if you react an alcohol with PBr3 and pyridine?
you replace the OH with Br
this only works with alcohols bonded to methyl carbons, primary carbons, or secondary carbons because this happens via Sn2
how will stereochemistry change when you react an alcohol with PBr3 and pyridine?
if there's a stereocenter on the carbon attached to the alcohol, this will flip the stereochemistry
what happens when you react an alcohol with SOCl2 and pyridine?
you replace the OH with Cl
this only works with alcohols bonded to methyl carbons, primary carbons, or secondary carbons because this happens via Sn2
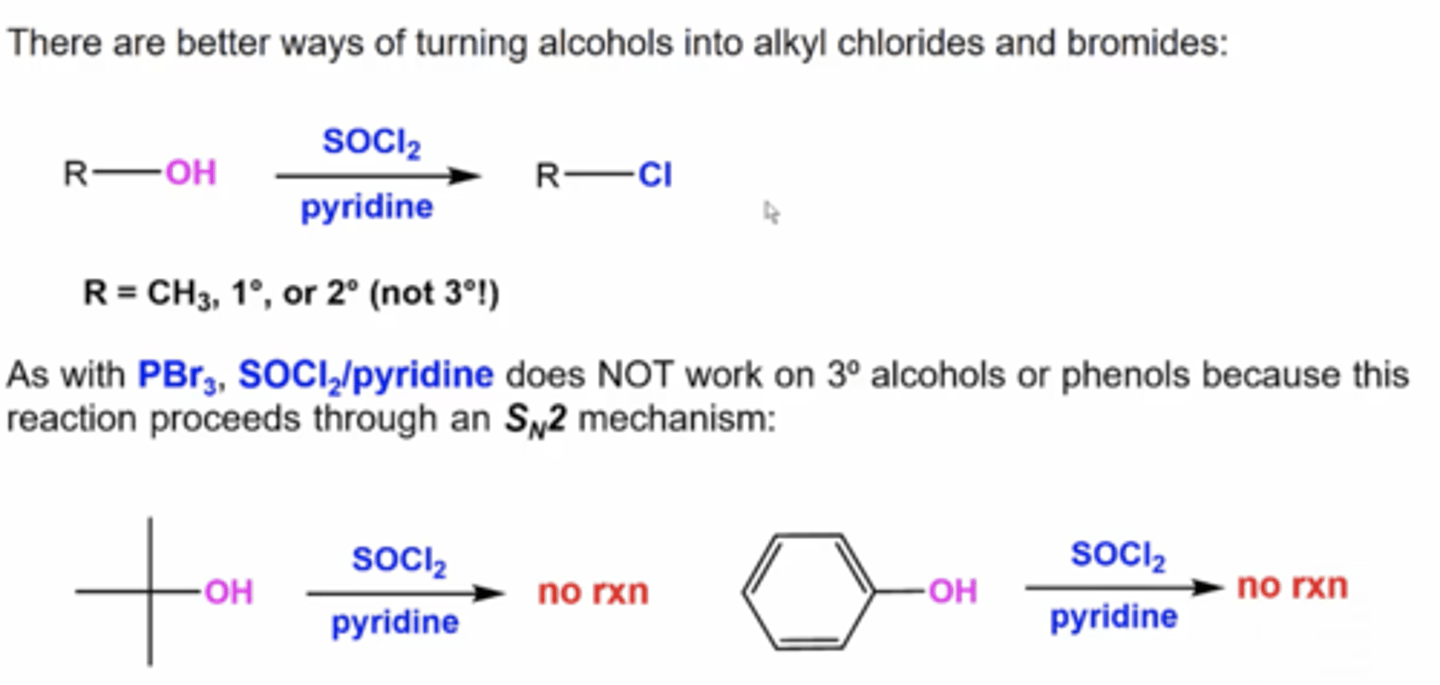
how will stereochemistry change when you react an alcohol with SOCl2 and pyridine?
if there's a stereocenter on the carbon attached to the alcohol, this will flip the stereochemistry
how can you make an OH a better leaving group?
react it with either TsCl + pyridine or MsCl + pyridine
this will convert your OH into OTs or OMs, which are good leaving groups
this has no effect on stereochemistry
what happens when you react OTs or OMs compounds with CH3S-?
the SCH3 will replace OTs/OMs and flip any stereochemistry if there's a chiral center
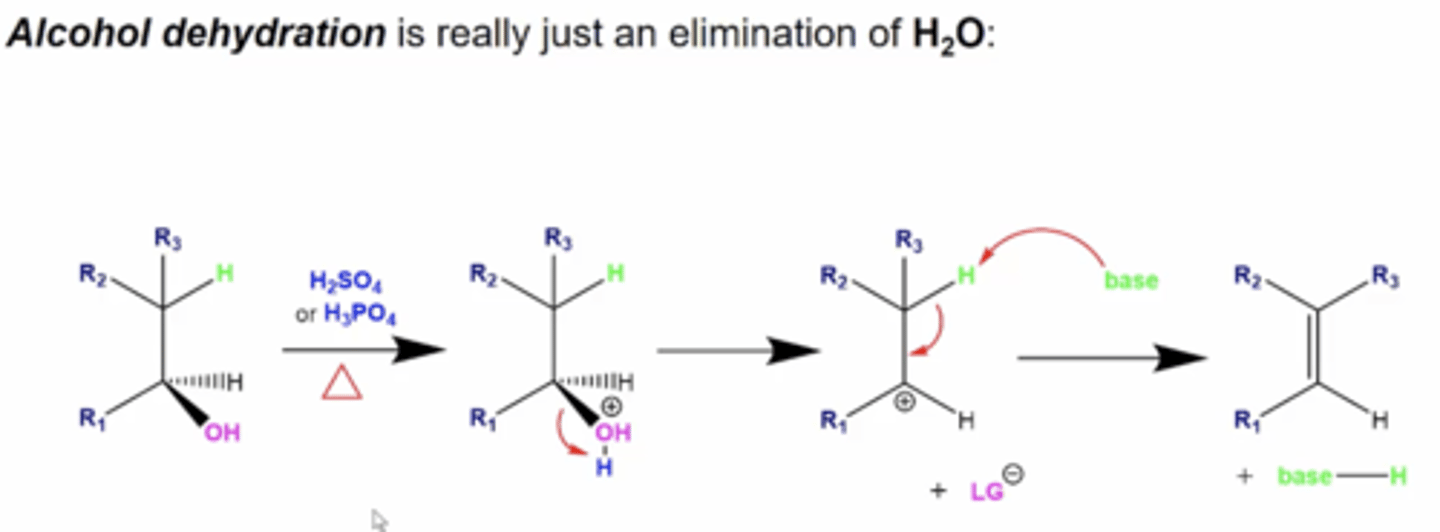
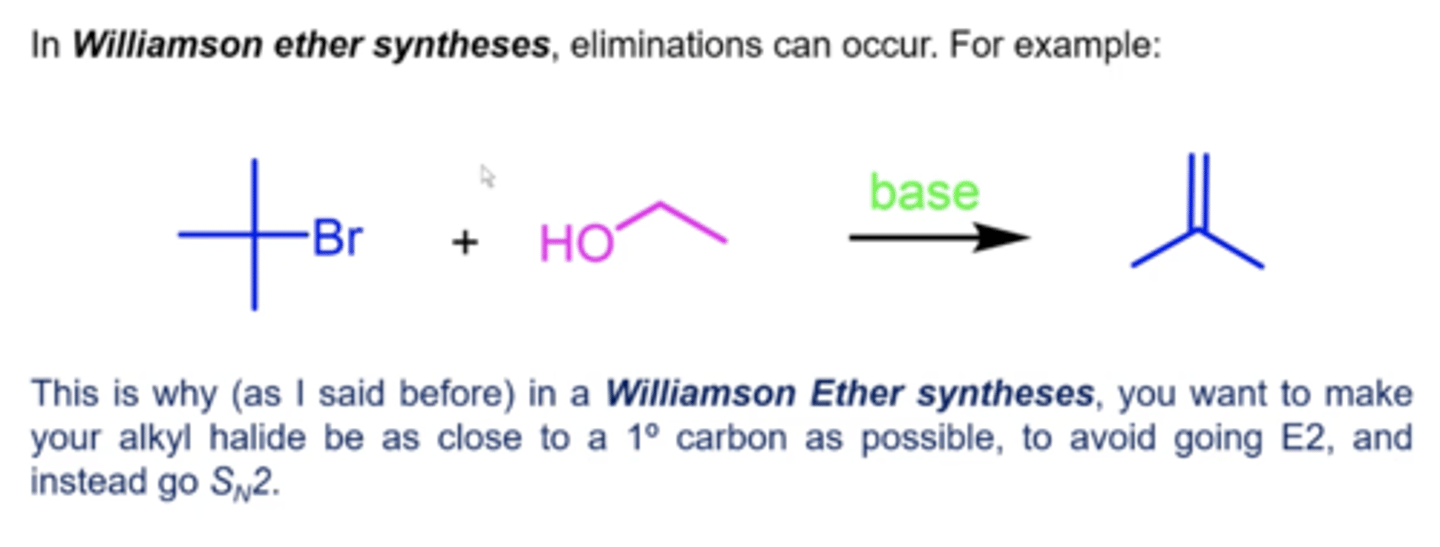
how can you dehydrate alcohols?
really, you're just performing an elimination (E1 or E2) reaction:
1. treat the alcohol with acid (H2SO4 or H3PO4) and heat
2. this will protonate OH to OH2 (water), which will leave, forming a carbocation intermediate
3. a weak base will now deprotonate another H, giving you the final alkene product
which elimination mechanism will primary alcohols undergo?
E2
(typically favors more substituted aka Zaitsev product)
which elimination mechanism will secondary and tertiary alcohols undergo?
E1
(typically favors more substituted aka Zaitsev product)
(these can also undergo carbocation rearrangements)
what reagents perform oxidation?
Na2Cr2O7/H2SO4, or H2CrO4, or CrO3/H2SO4
what happens if you treat a primary alcohol with Na2Cr2O7/H2SO4, or H2CrO4, or CrO3/H2SO4?
it oxidizes the carbon attached to the alcohol to become a carboxylic acid
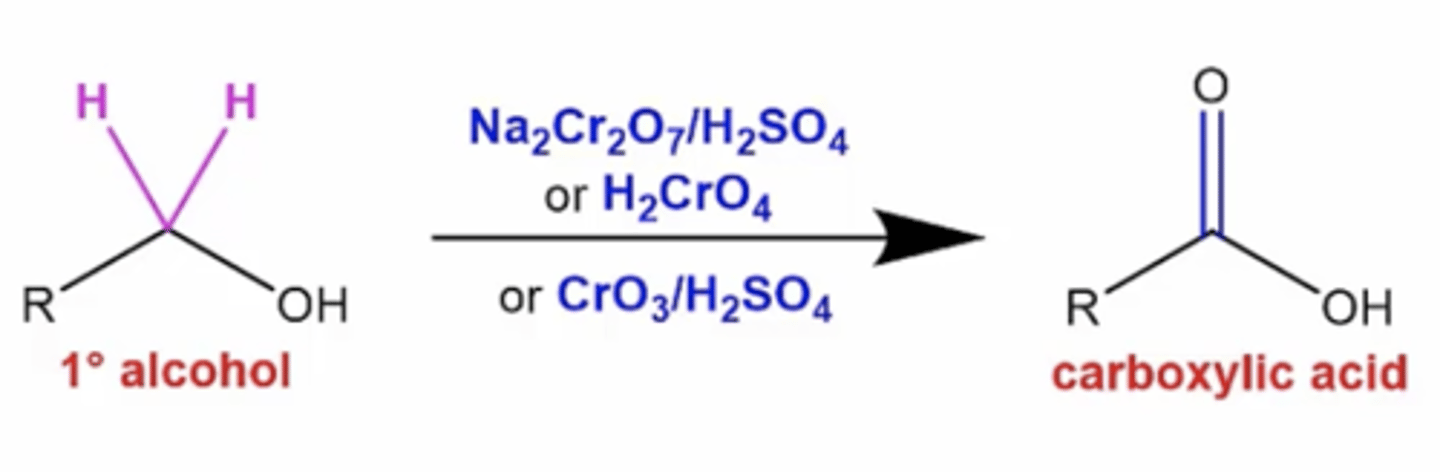
what happens if you treat a secondary alcohol with Na2Cr2O7/H2SO4, or H2CrO4, or CrO3/H2SO4?
it oxidizes the carbon attached to the alcohol to become a ketone
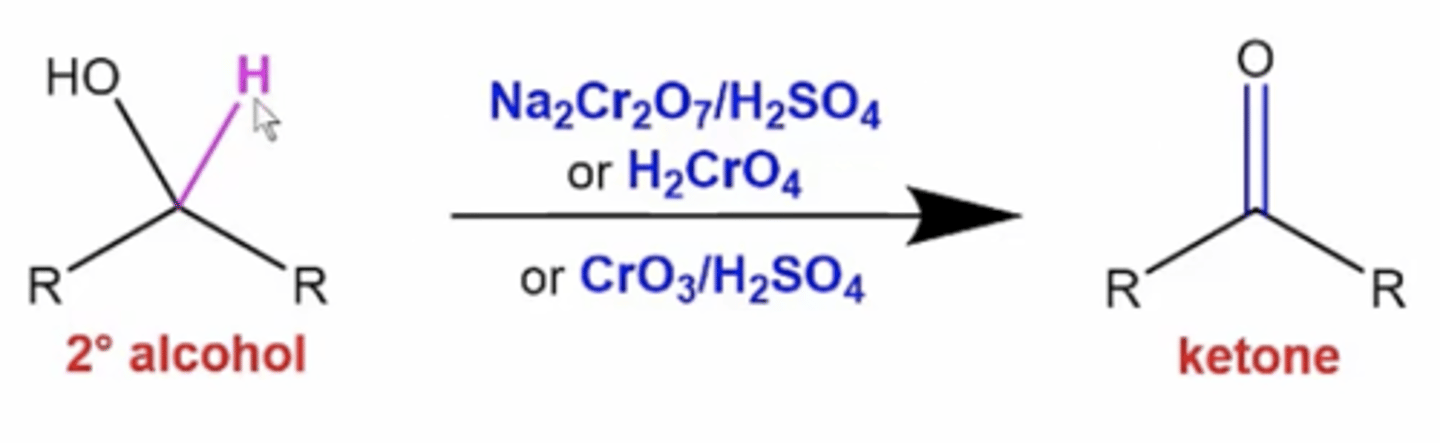
what happens to tertiary alcohols or phenols when you react them with oxidizing reagents like Na2Cr2O7/H2SO4, or H2CrO4, or CrO3/H2SO4?
no reaction because tertiary alcohols and phenols don't have any hydrogens to oxidize
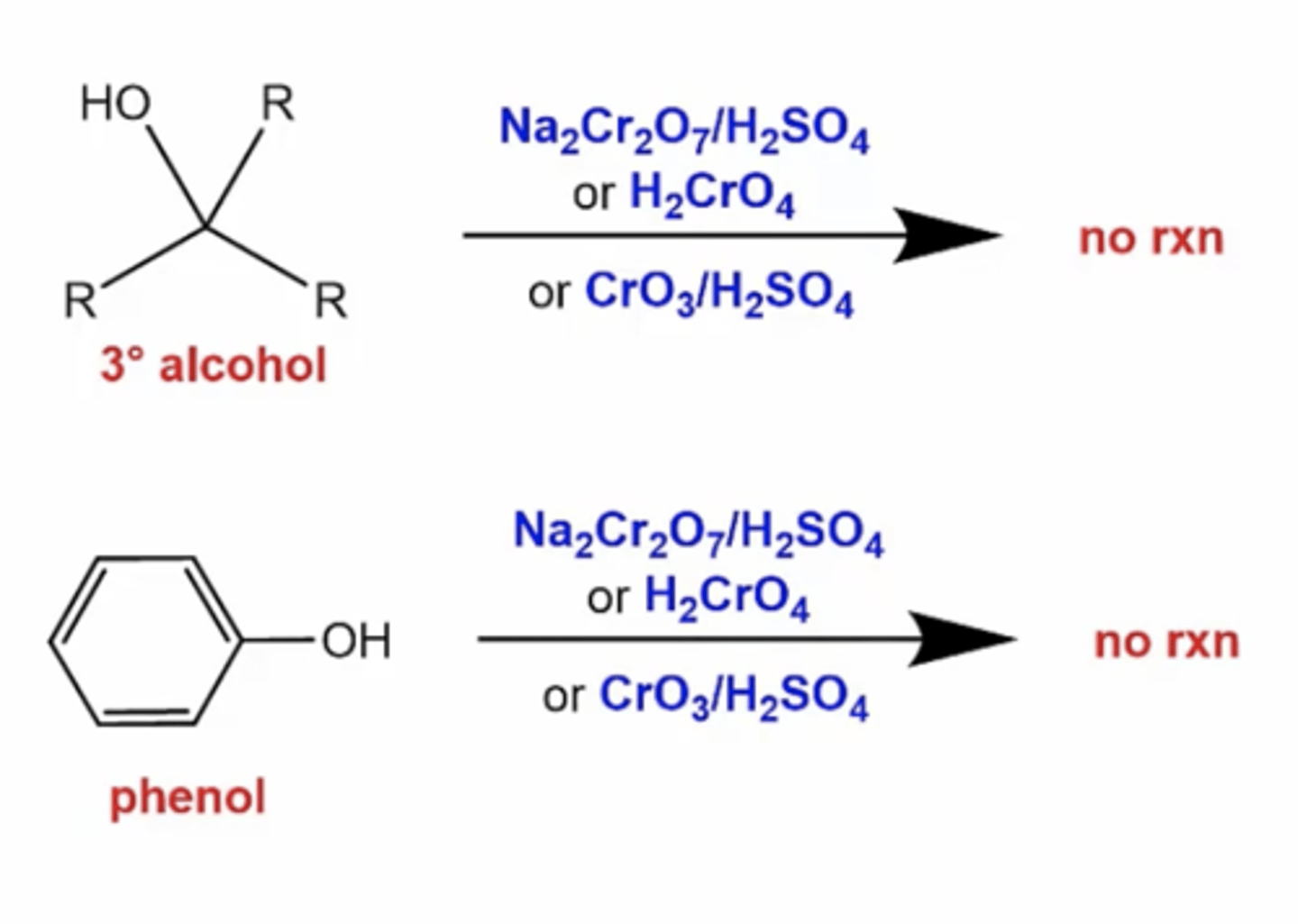
how can you convert a primary alcohol into an aldehyde so that it's not completely oxidized?
use PCC or periodinane
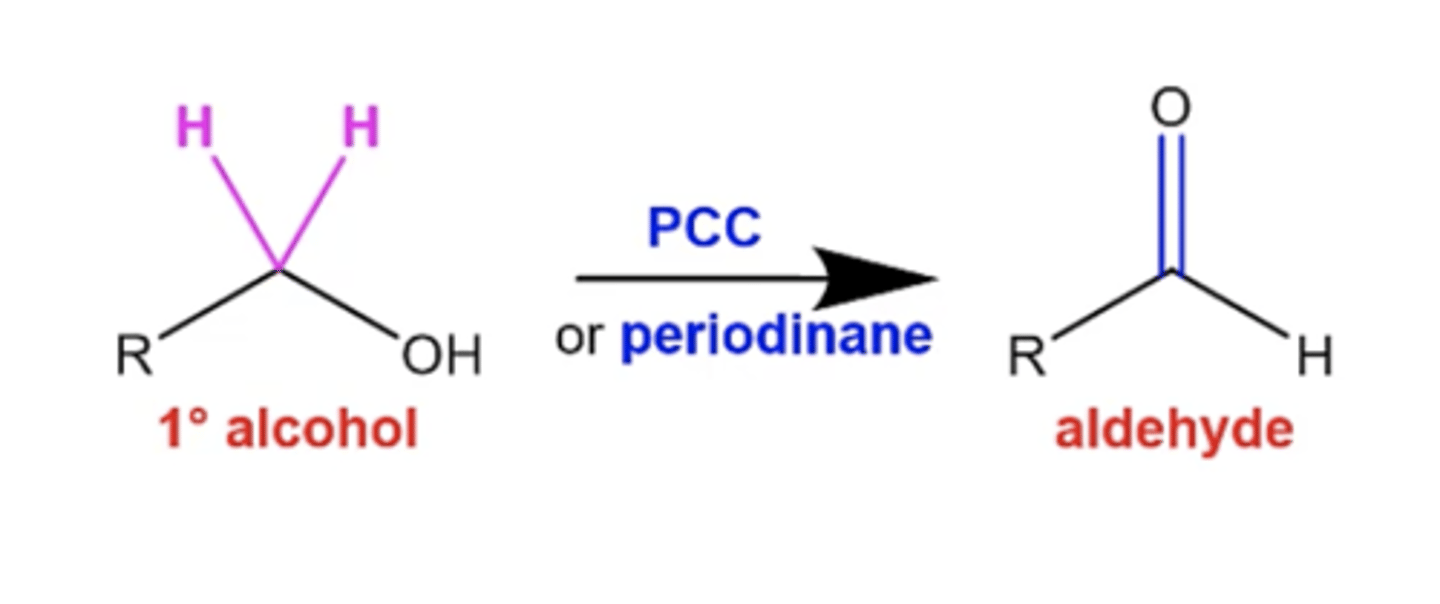
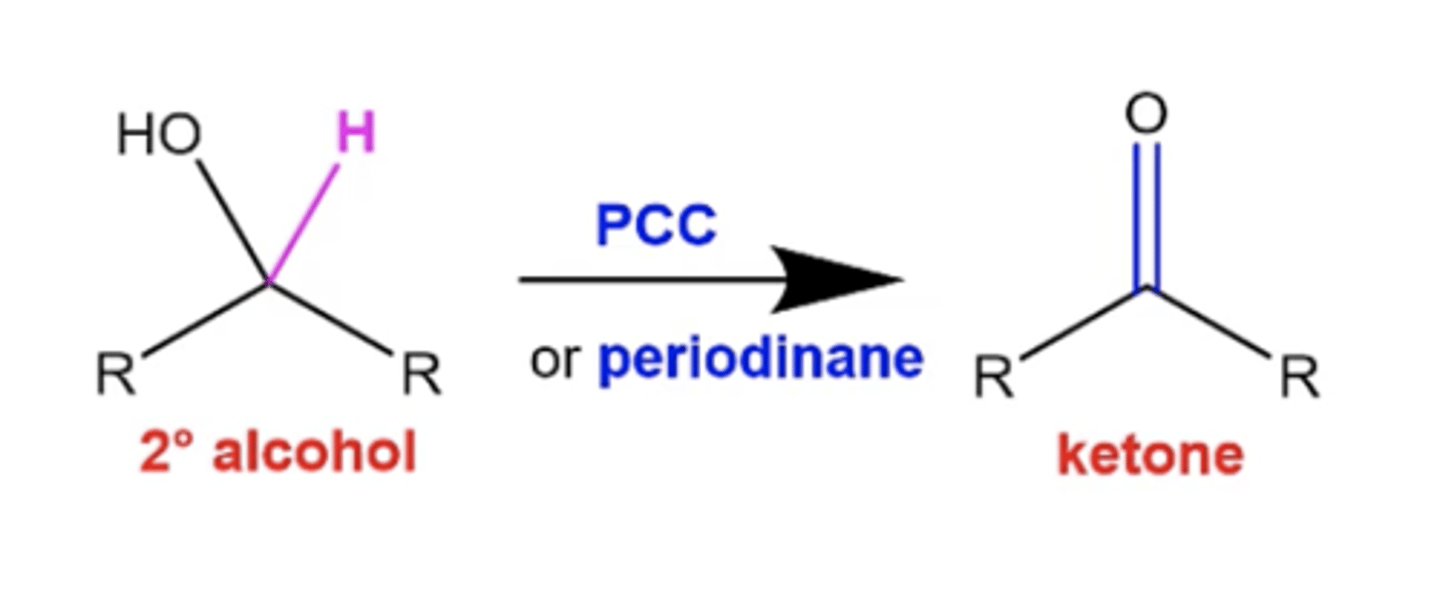
what happens if you treat a secondary alcohol with PCC or periodinane?
it converts it to a ketone, just like Na2Cr2O7/H2SO4, or H2CrO4, or CrO3/H2SO4
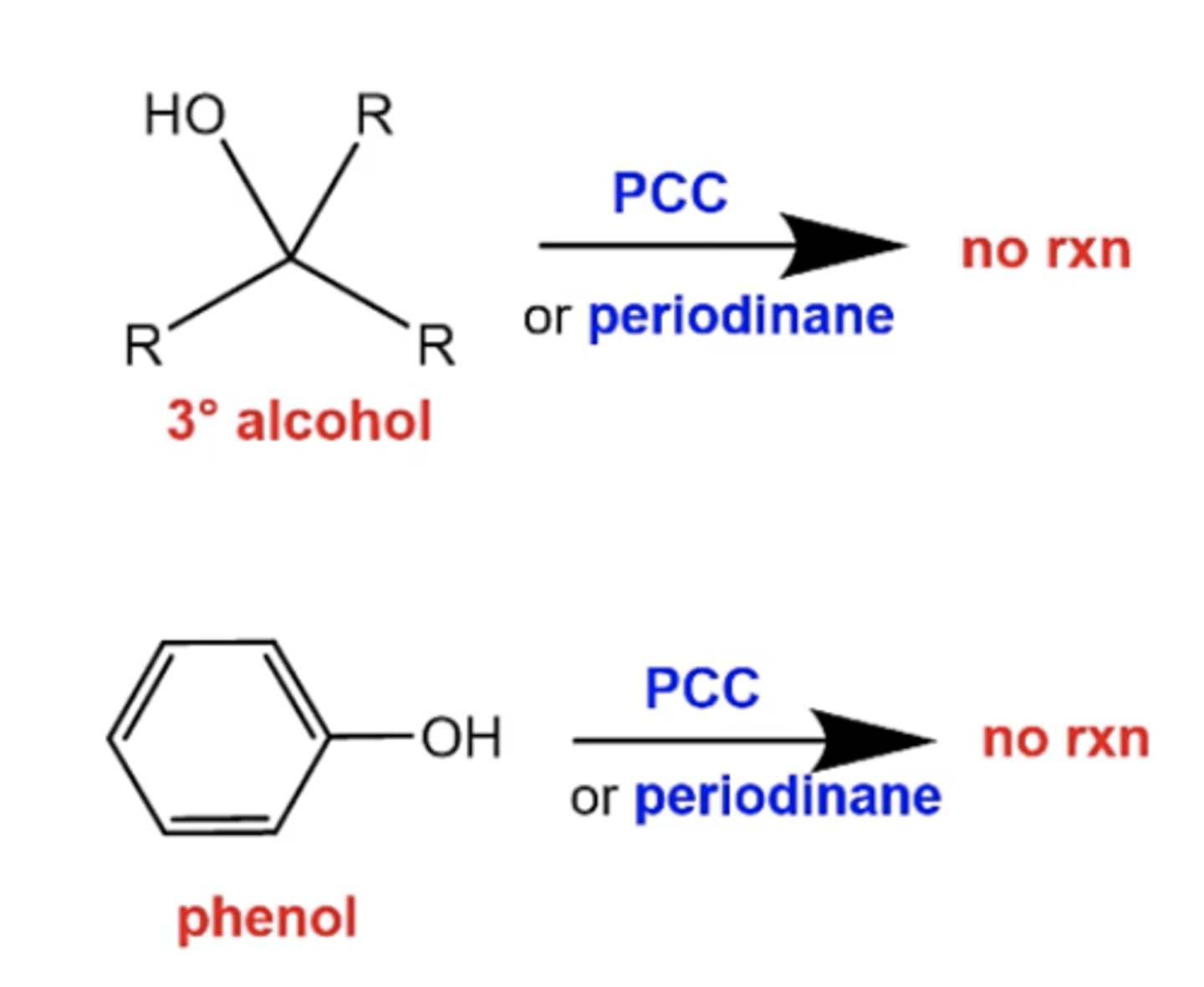
what happens if you react a tertiary alcohol or phenol with PCC or periodinane?
again, no rxn because there's no hydrogens to oxidize
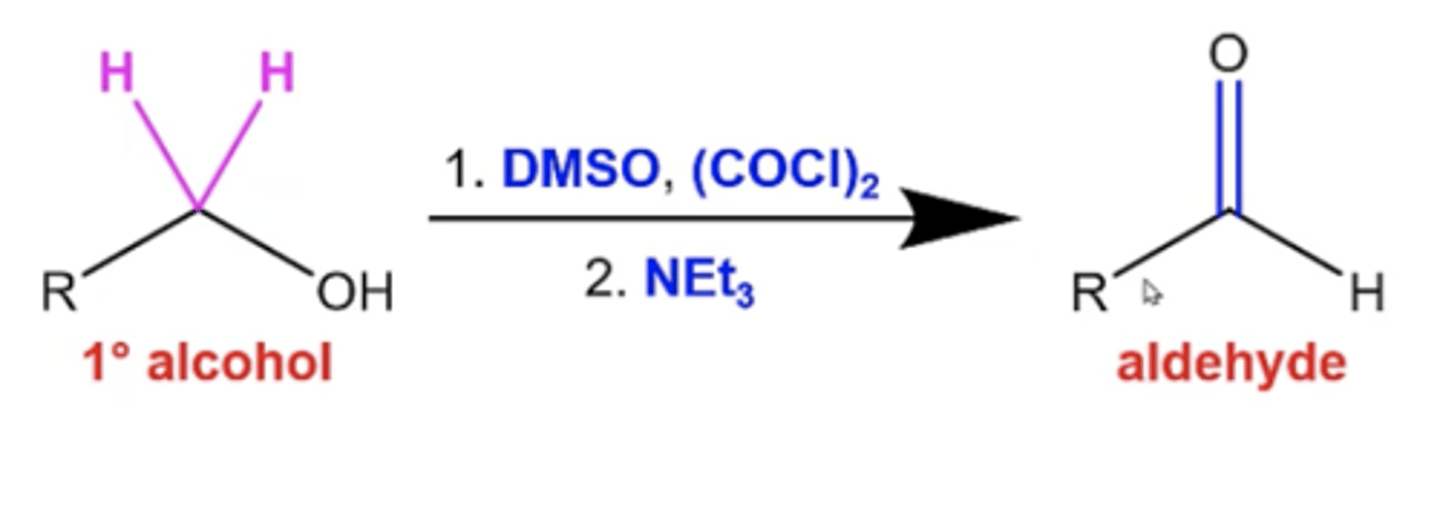
how do you perform a conversion between a primary alcohol and an aldehyde via Swern oxidation?
use these reagents:
1. DMSO, (COCl)2
2. NEt3
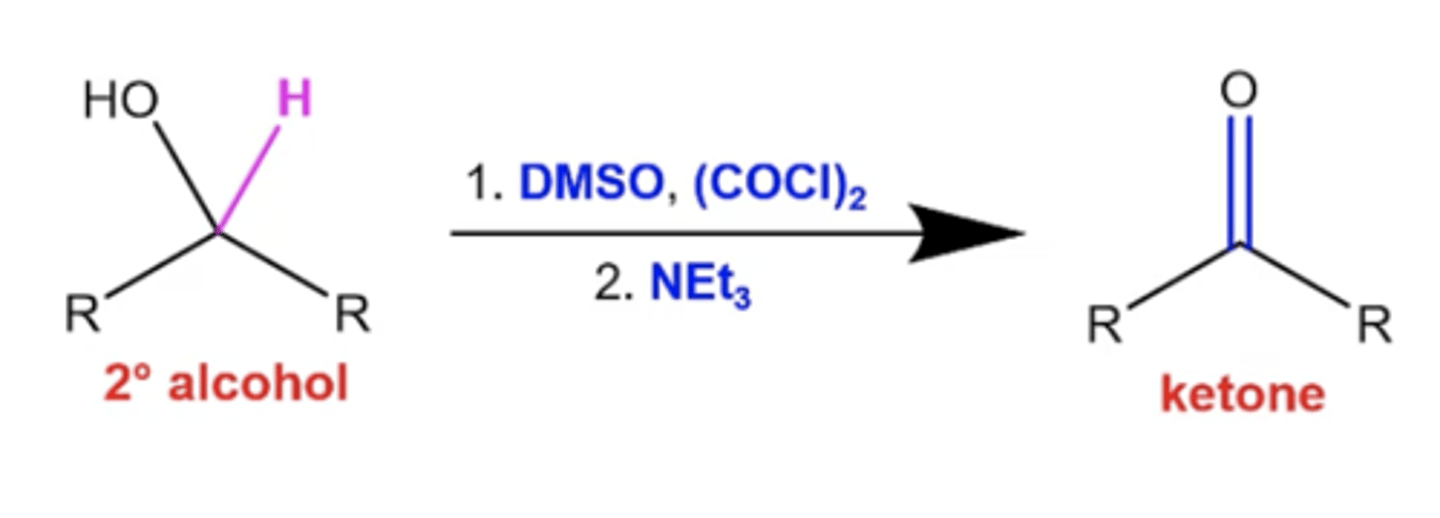
how do you perform a conversion between a secondary alcohol and a ketone via Swern oxidation?
use these reagents:
1. DMSO, (COCl)2
2. NEt3
how do you synthesize ethers using Williamson ether synthesis?
-start with an alcohol and react it with these reagents to deprotonate the alcohol:
NaH, Na, or K
-react the deprotonated alcohol with an alkyl halide
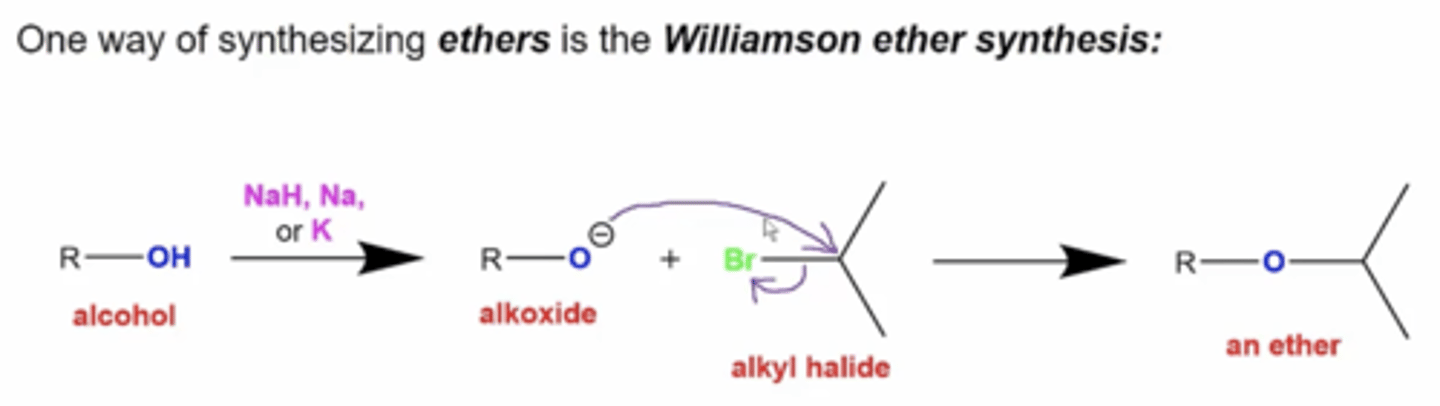
what are the three reactants necessary for a Williamson ether synthesis?
1. alkyl halide (as close to a primary one as possible)
2. alcohol
3. base (NaH, Na, K, or even NaOH)
which mechanism does Williamson ether synthesis go by?
it goes via Sn2, so it will give an inverted stereochemistry if applicable
if given an ether, how would you compose the reactants to make that ether?
split up the ether to make the alkyl halide (the one with a halogen) ›as close to a primary carbon as possible, to encourage an Sn2 mechanism
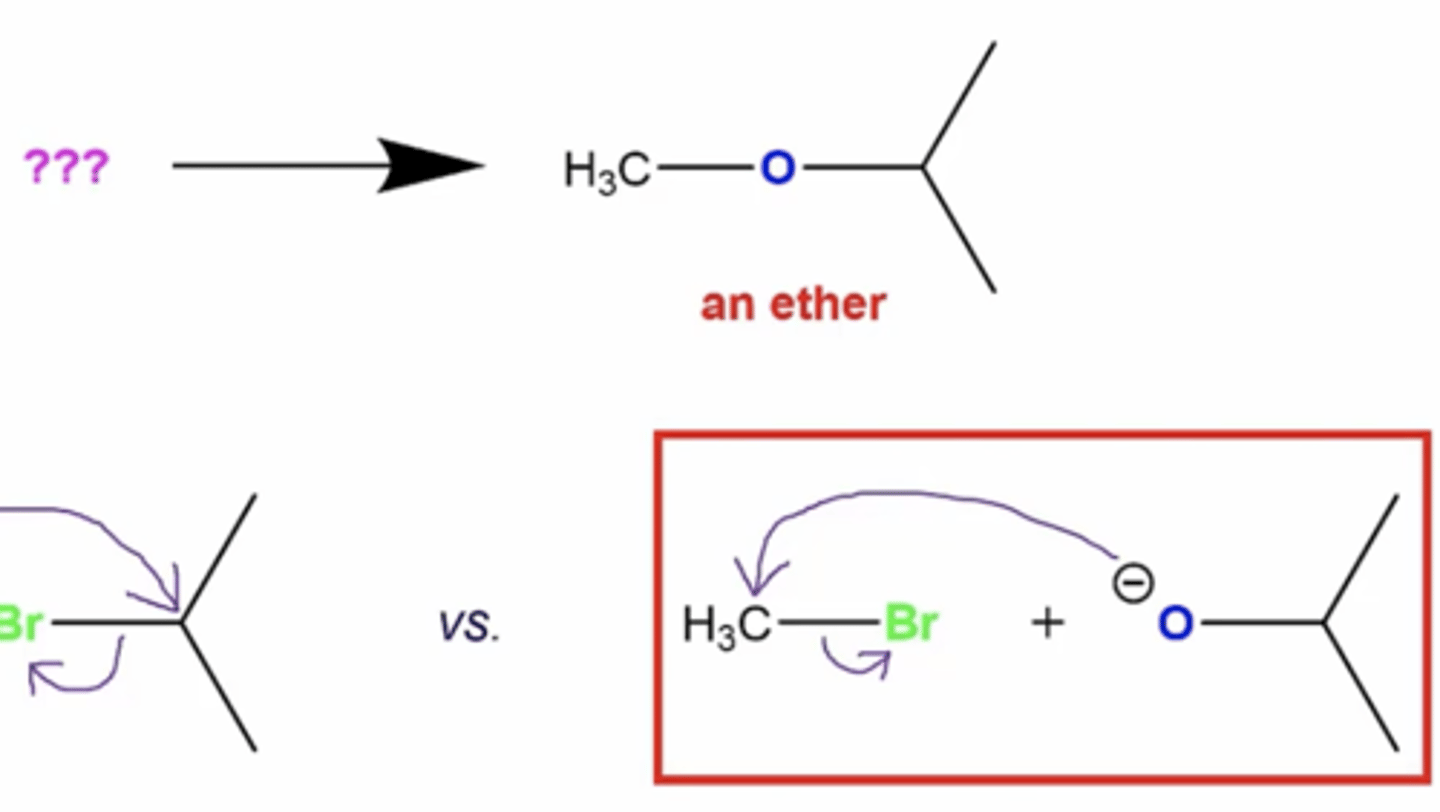
how might a Williamson ether synthesis actually go E2 to form an alkene vs. Sn2 to form an ether?
if you have a tertiary alkyl halide, the alcohol cannot attack the tertiary carbon because of steric hindrance, so you go E2 style instead:
-this is where the base deprotonates the alcohol, and the base will instead deprotonate a neighboring hydrogen to form an alkene
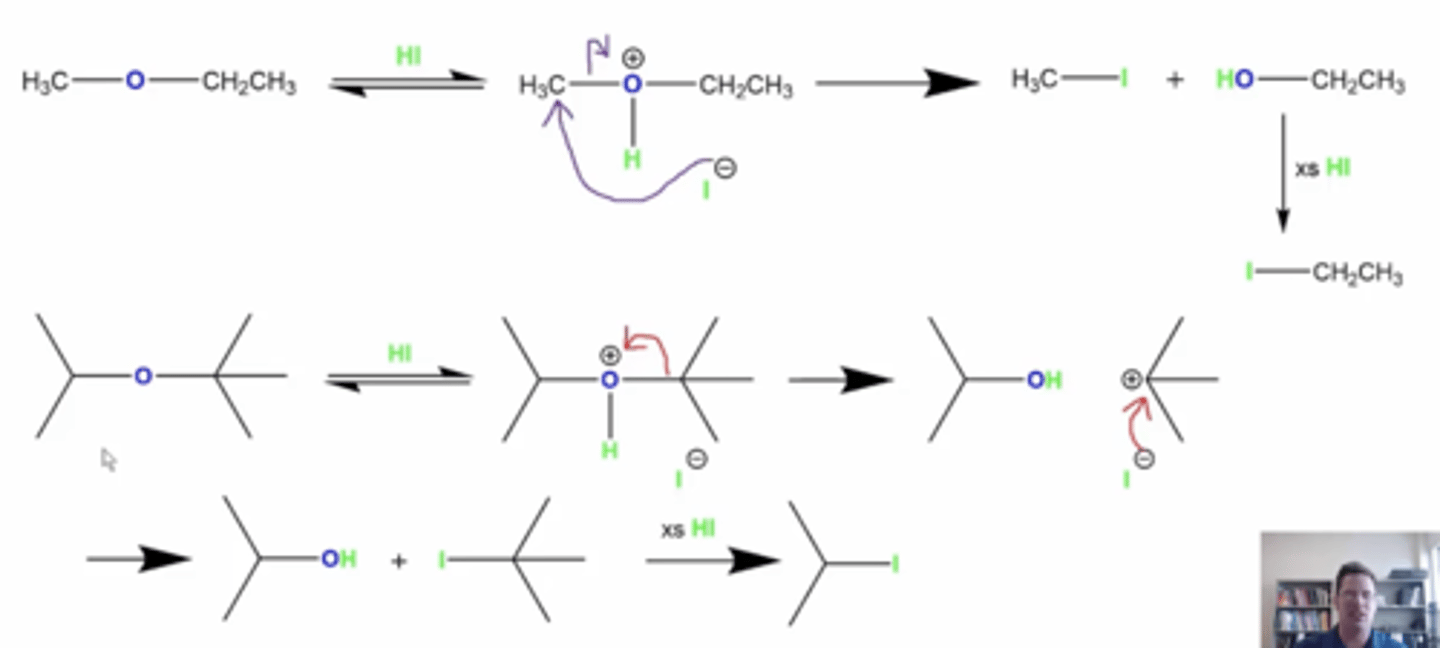
what happens if you react ethers with HX (X=Cl, Br, or I)?
it will split the ether into an alkyl halide and an alcohol
if you add excess HX, then you get two alkyl halides
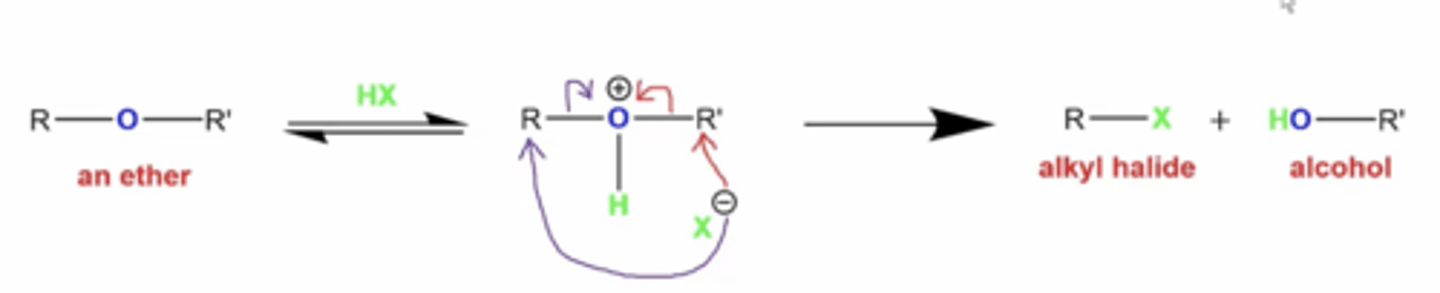
how do you determine which R group the halogen attacks?
-if any R groups are secondary or tertiary carbon groups, these will form stable carbocations and the reaction will go through Sn1 mechanism
-if there are R groups that are methyl or primary carbon groups, they will not form stable carbocations and the reaction will go through Sn2 mechanism
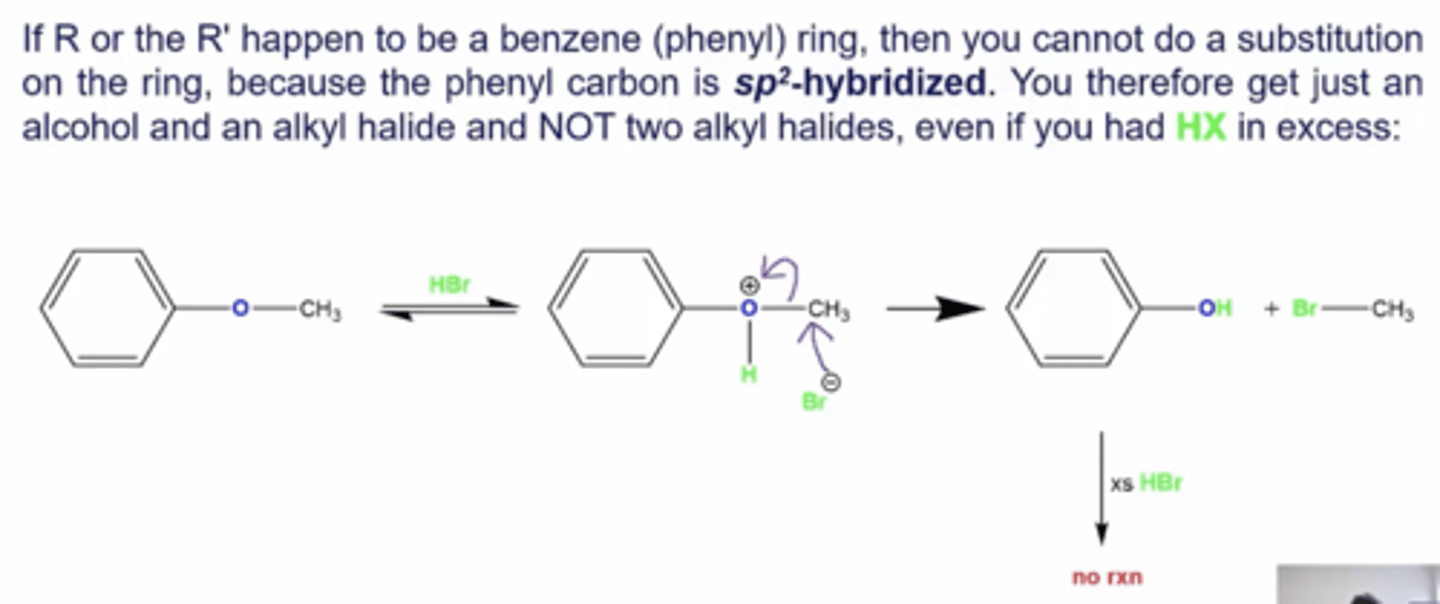
what happens if you react an ether with HX, but one of the R groups happen to be a phenyl ring?
-you can't do a substitution on the ring because SN1/2 reactions cannot be done on Sp2 hybridized carbons, so instead you'll just get an alcohol and alkyl halide, regardless of whether you have HX in excess
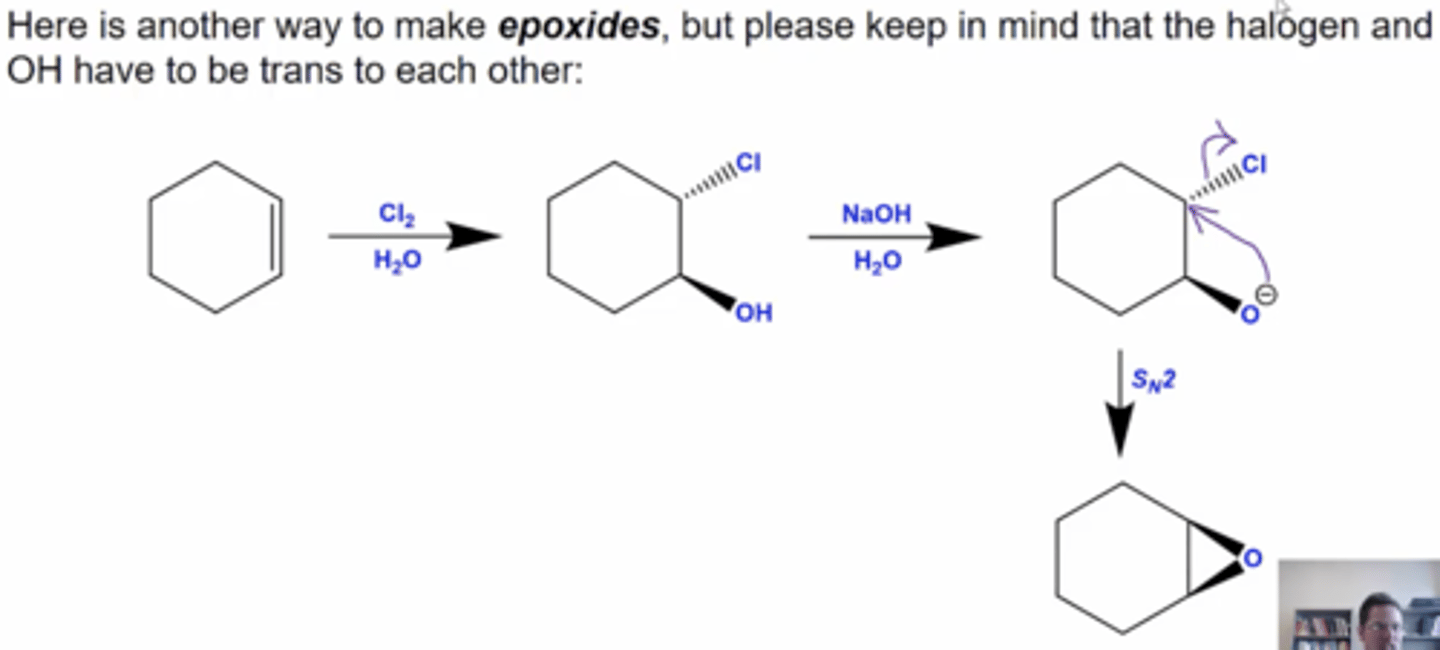
how can you convert an alkene ring into an epoxide?
1. react the ring with Cl2 and H2O, which will add OH and Cl trans to each side of the alkene
2. react this new ring with NaOH and H2O to form the epoxide via Sn2 mechanism
the halogen and OH must be trans to each other
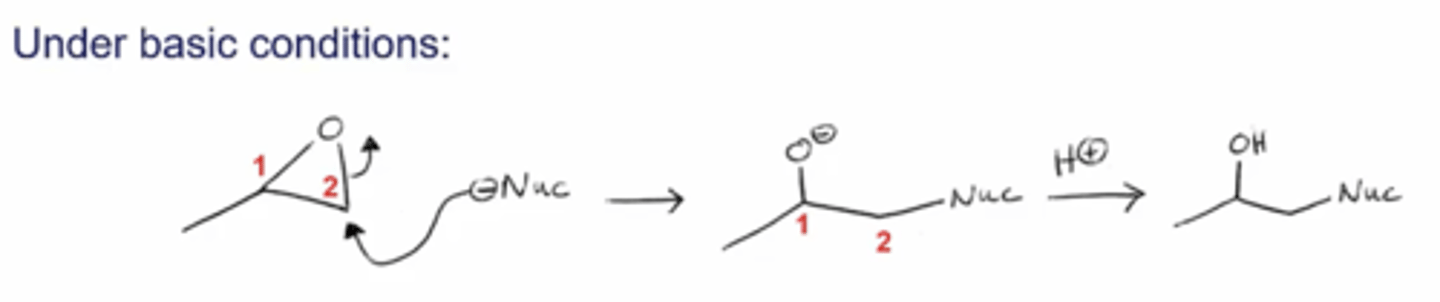
what happens if you react an epoxide with a nucleophile under basic conditions?
the nucleophile will attack the carbon of the epoxide that's less substituted, opening the epoxide and producing an OH on the more substituted side and a Nu on the less substituted side
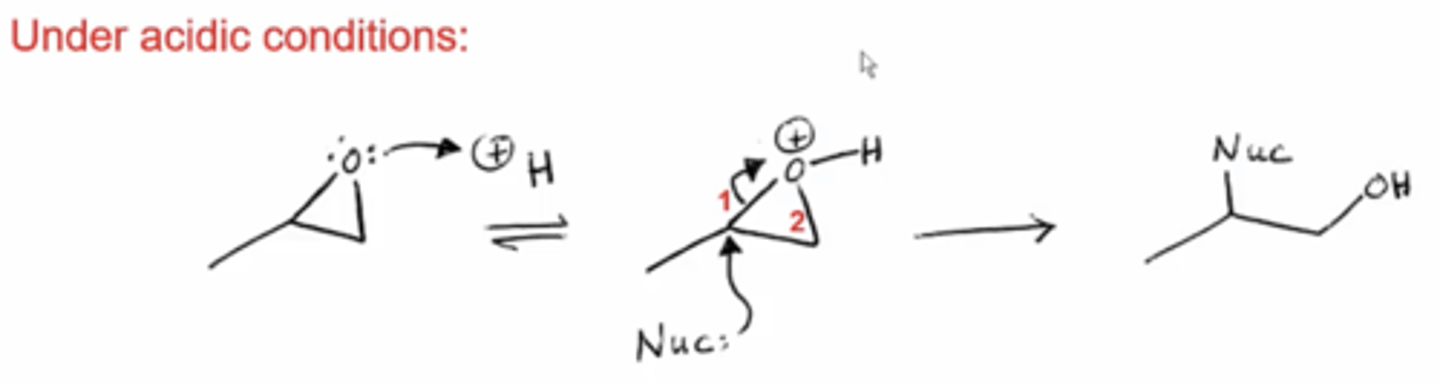
what happens if you react an epoxide with a nucleophile under acidic conditions?
the lone pairs on the epoxide's O will attack an H in the acidic solution
this will break the epoxide on the side that will produce the more stable carbocation (the more substituted side)
the weak Nucleophile will then attack the epoxide at the more substituted side and open it up, producing an OH on the less substituted side and a Nu on the more substituted side
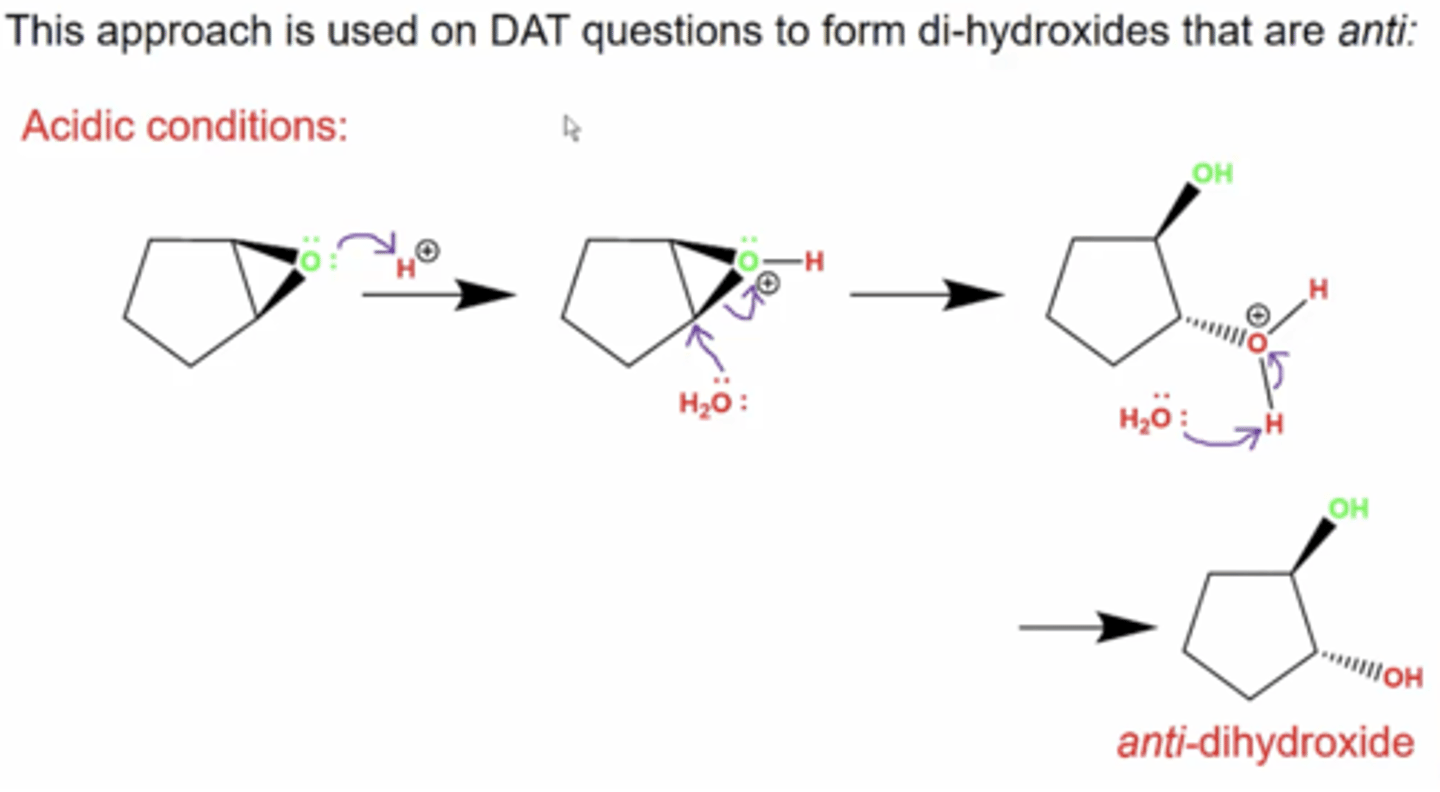
what is produced if you react a ring epoxide under either basic or acidic conditions?
you produce an anti dihydroxide either way
how can you open a ring epoxide to produce a syn dihydroxide?
you have to take an alkene ring and react it with OsO4