Chemistry II Lesson 8: Aldehydes and Ketones Part I
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
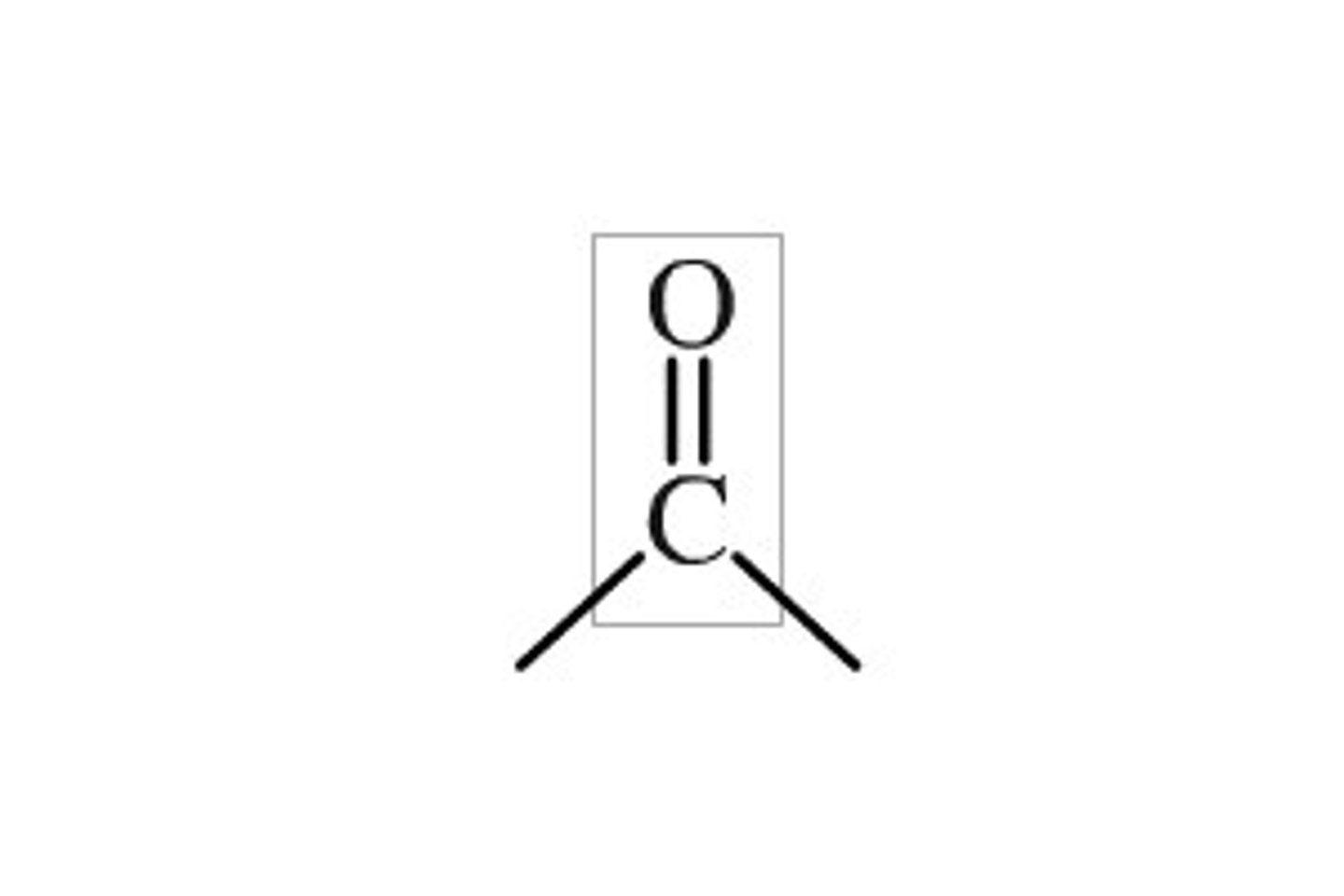
CRB Draw a generic carbonyl functional group.
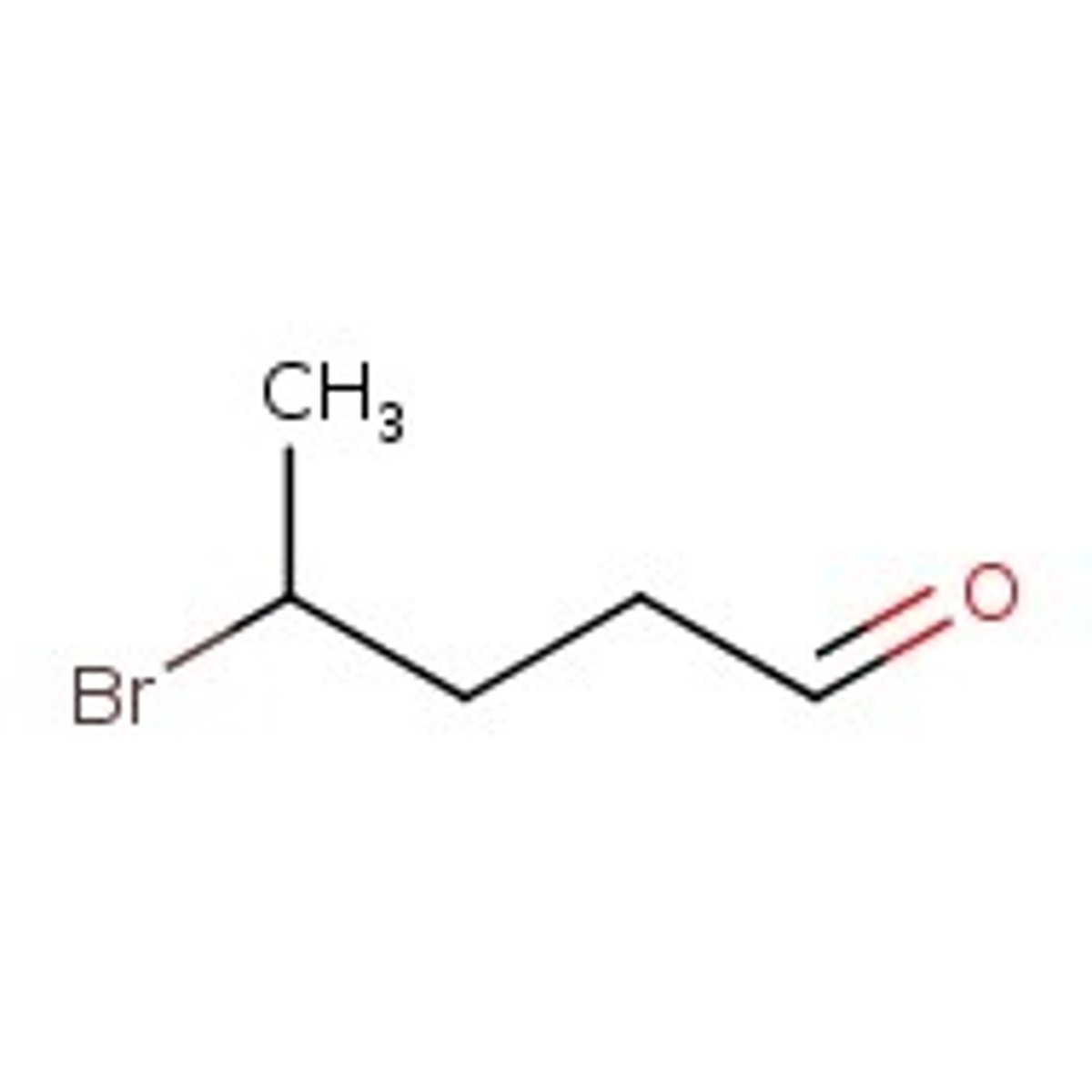
Draw 4-bromopentanal.
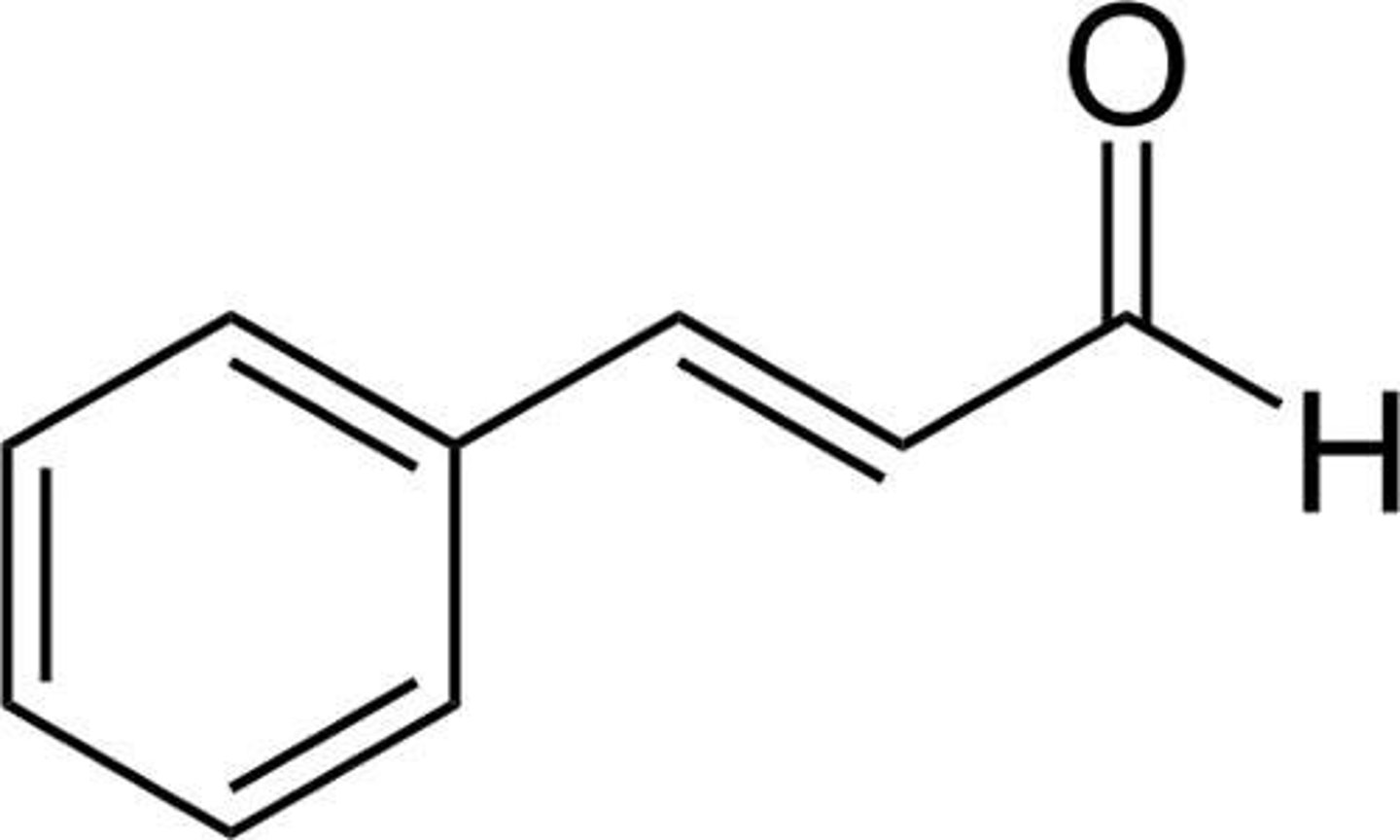
Draw (E)-3-phenyl-2-propenal (cinnamaldehyde).
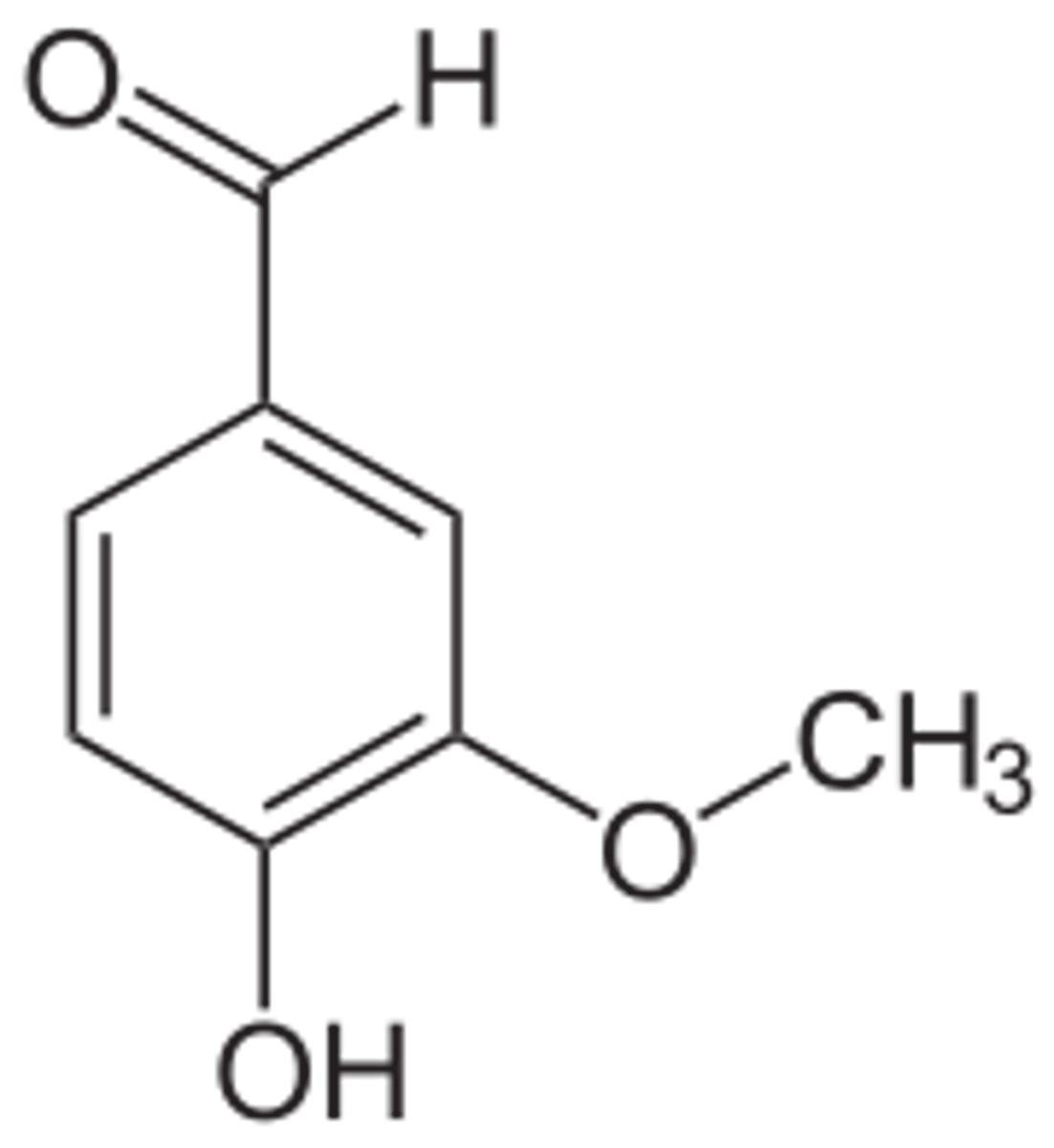
Draw 4-hydroxy-3-methoxy benzaldehyde (vanillin).
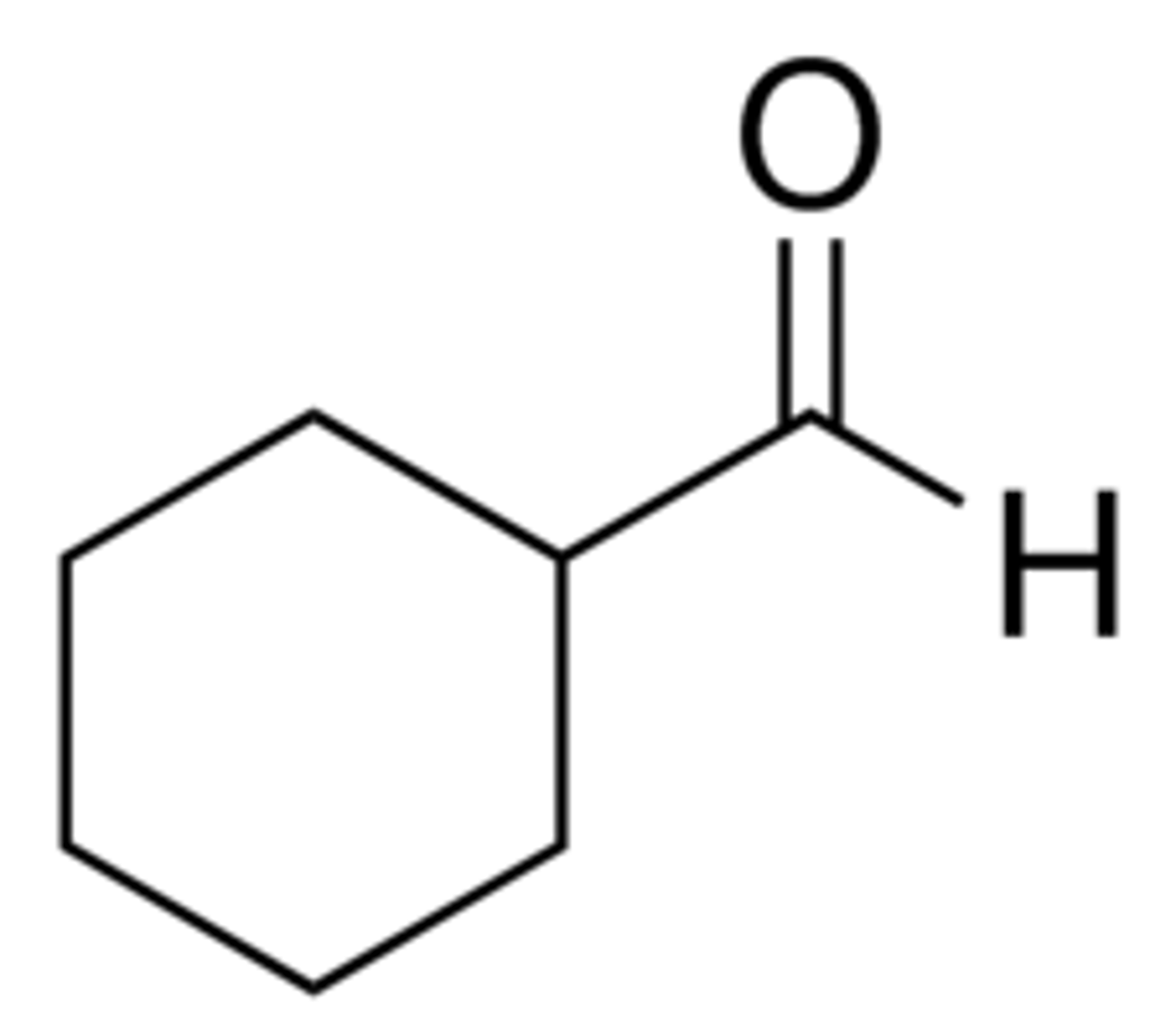
Draw Cyclohexane Carbaldehyde
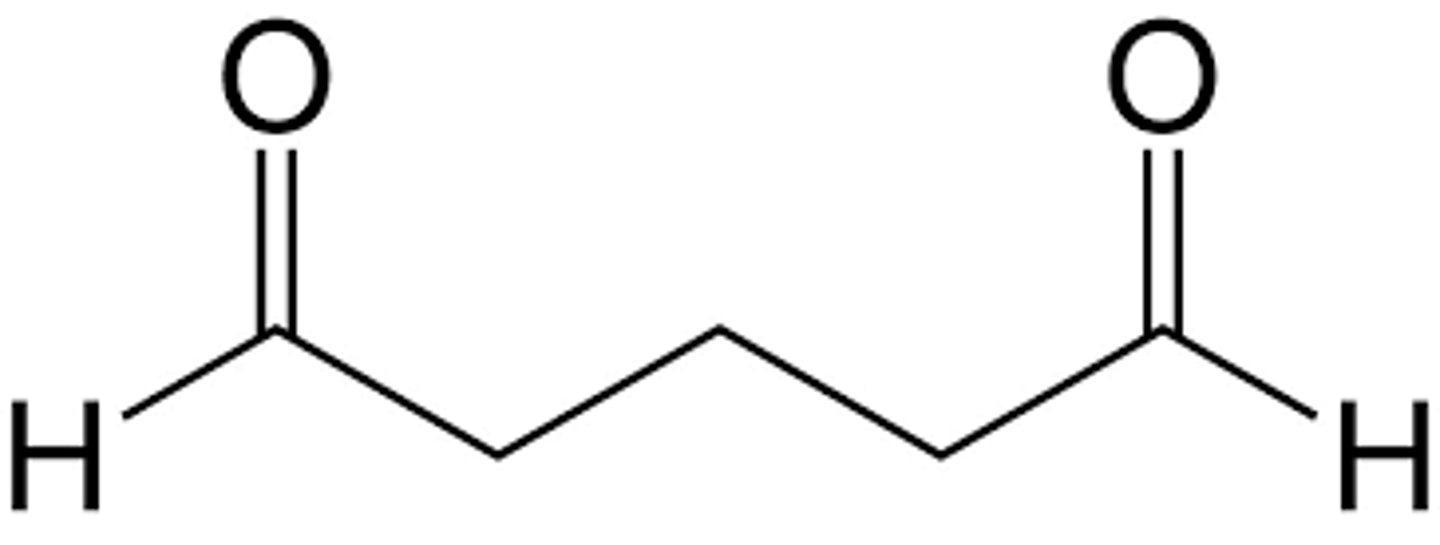
Draw 1,5-pentanedial
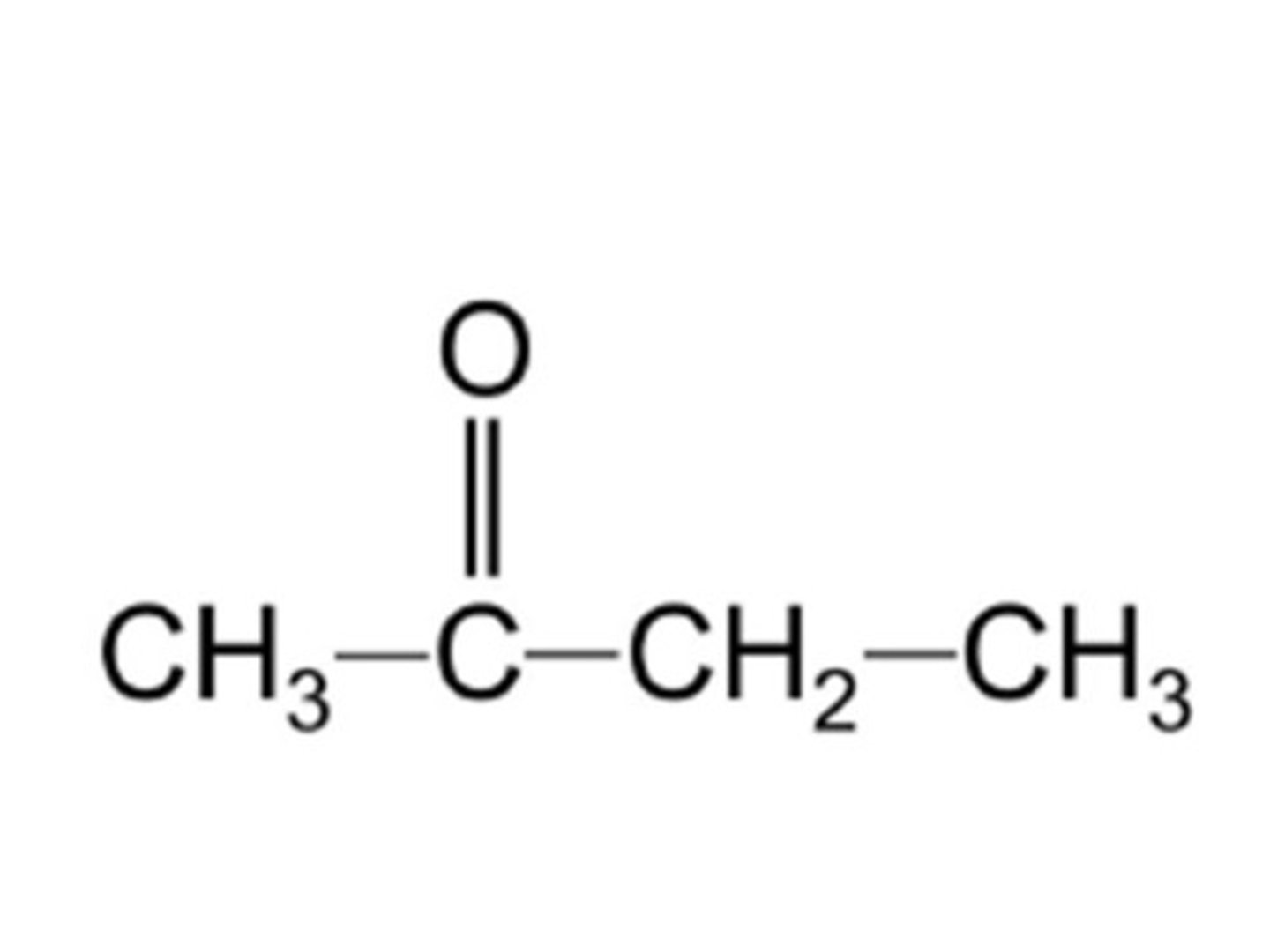
Draw butanone.
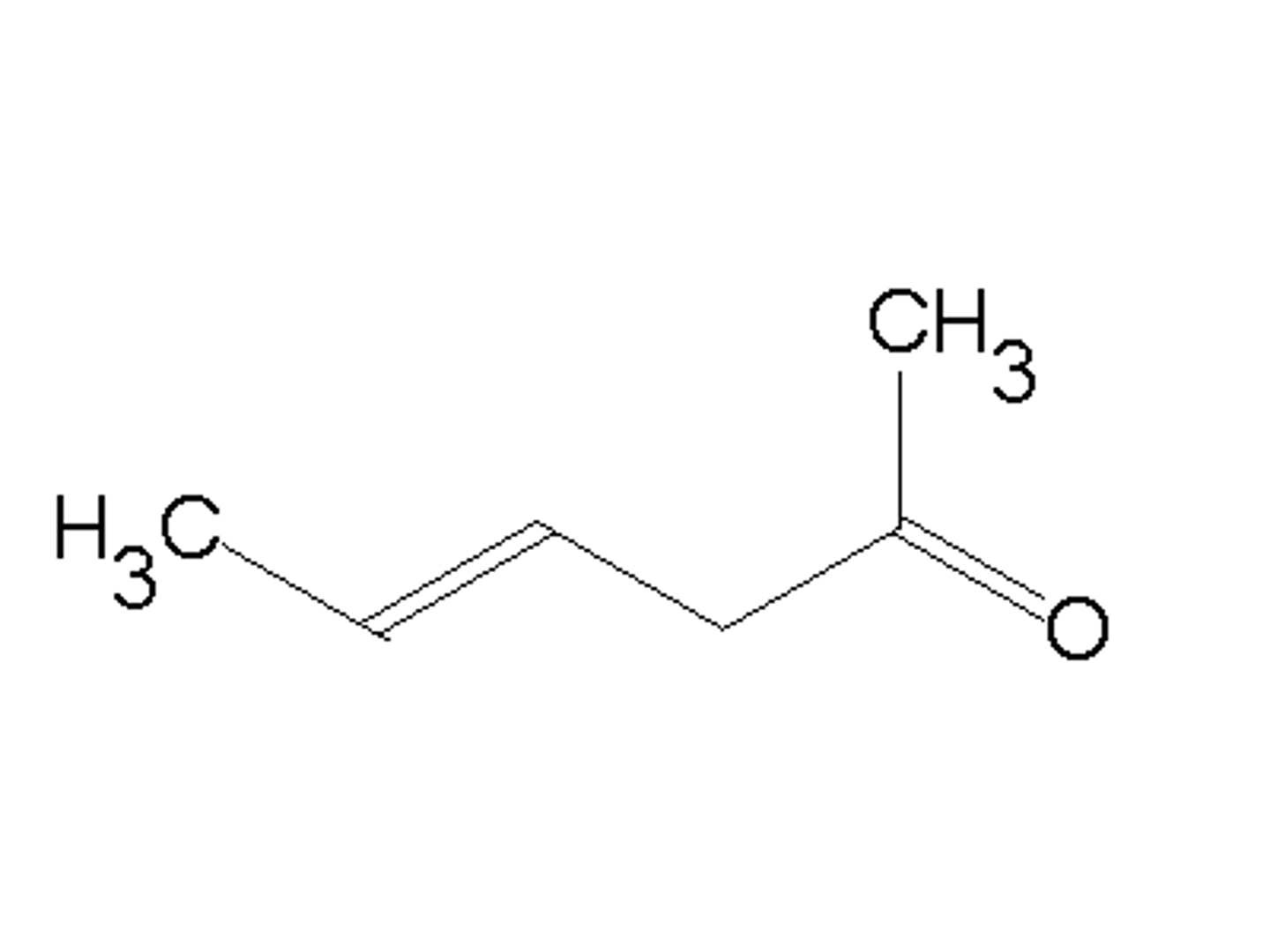
Draw (E)-4-hexen-2-one.
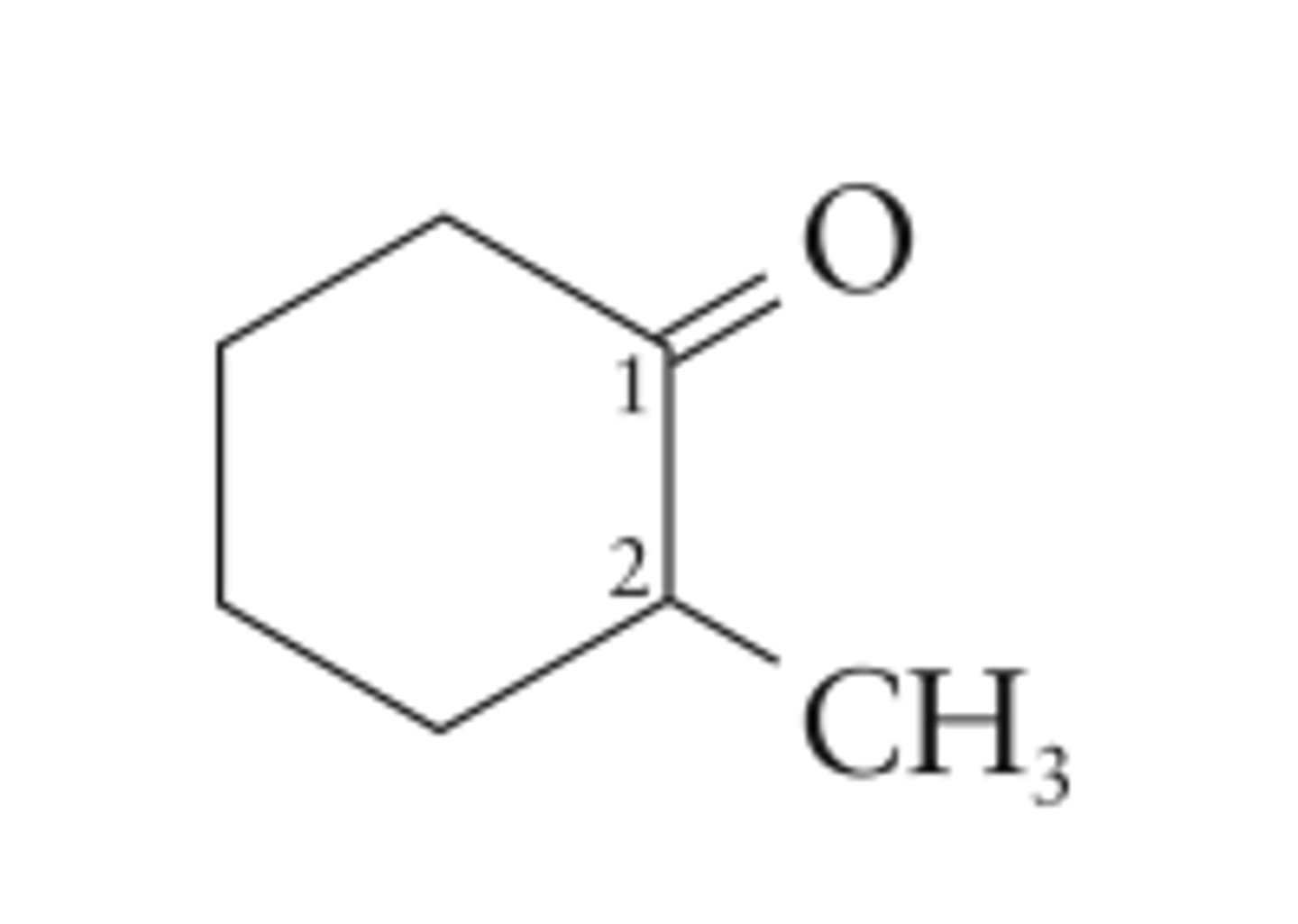
Draw 2-methylcyclohexanone.
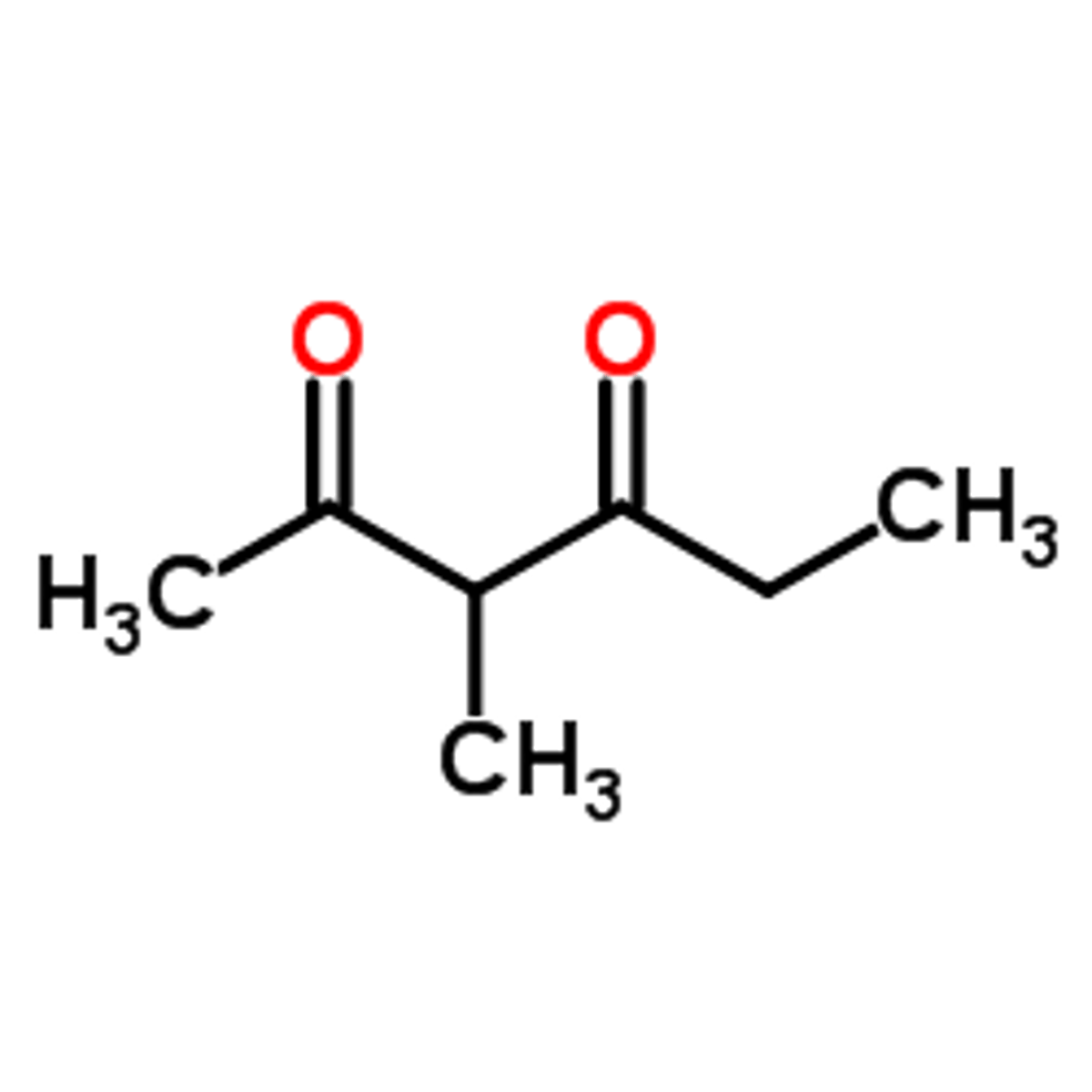
Draw 3-methyl-hexane-2,4-dione.
The word "aldehyde" comes from the two words alcohol and dehydrogenatum. What's the connection here?
Aldehydes are formed via the dehydrogenation of alcohols.
CRB If a strong oxidizing agent like Cr2O7 is mixed with an alcohol, will an aldehyde form?
(A) No, a reducing agent would turn an alcohol into an aldehyde.
(B) Yes, but only as a transition to a more oxidized carboxylic acid product.
(C) Yes, but only as a transition to a more oxidized ketone product.
(D) Yes, and the aldehyde is the final product.
(B) Yes, but only as a transition to a more oxidized carboxylic acid product.
A strong oxidizing agent will be able to oxidize an alcohol to a carboxylic acid.
CRB Which of the following oxidizing agents would be able to convert an aldehyde to an alcohol?
(A) PCC
(B) PDC
(C) HIO4 (Periodic acid)
(D) None of the above
(D) None of the above
For the reaction in the question to occur, there must be a reducing agent! PCC, PDC and HIO4 are all weak oxidizing agents that can turn an alcohol into an aldehyde/ketone.
CRB What will a strong oxidizing agent do to a secondary alcohol?
(A) It will not react, since there is no more possible bonds to be made.
(B) It will react and create an ketone intermediate, which is quickly oxidized again to form a carboxylic acid.
(C) It will react and create an aldehyde as the final product.
(D) It will react and create a ketone as the final product.
(D) It will react and create a ketone as the final product.
Recall that a secondary alcohol has two carbons bound to the alcohol-carbon, so the first oxidation product must be a ketone. Then, there are no hydrogens to be oxidized, so no further reaction will occur.
The word "acetone" comes from the word aketon. What's the connection here?
Acetone is a ketone (A-keton).
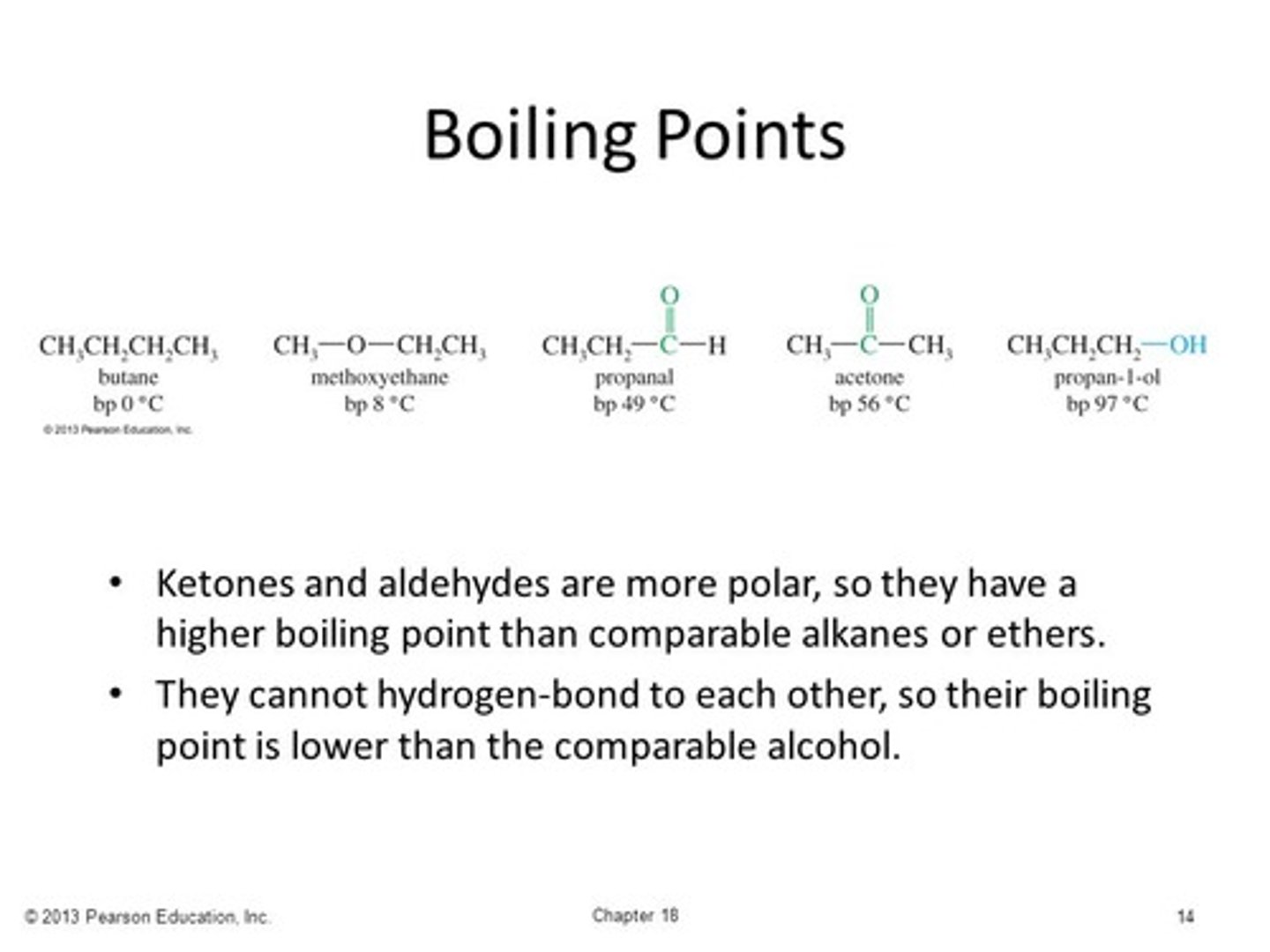
Order the following functional groups in order of increasing boiling point:
I. Isopropyl Alcohol
II. Acetone
III. Propane
IV. Propanal
(A) I < II < III < IV
(B) IV < III < II < I
(C) IV < II < III < I
(D) III < IV < II < I
(D) III < IV < II < I
In order of increasing boiling point: Propane (London Dispersion Forces only) < Propanal (Dipole-dipole Interactions) < Acetone (Dipole-dipole Interactions) < Isopropyl Alcohol (Hydrogen Bonding)
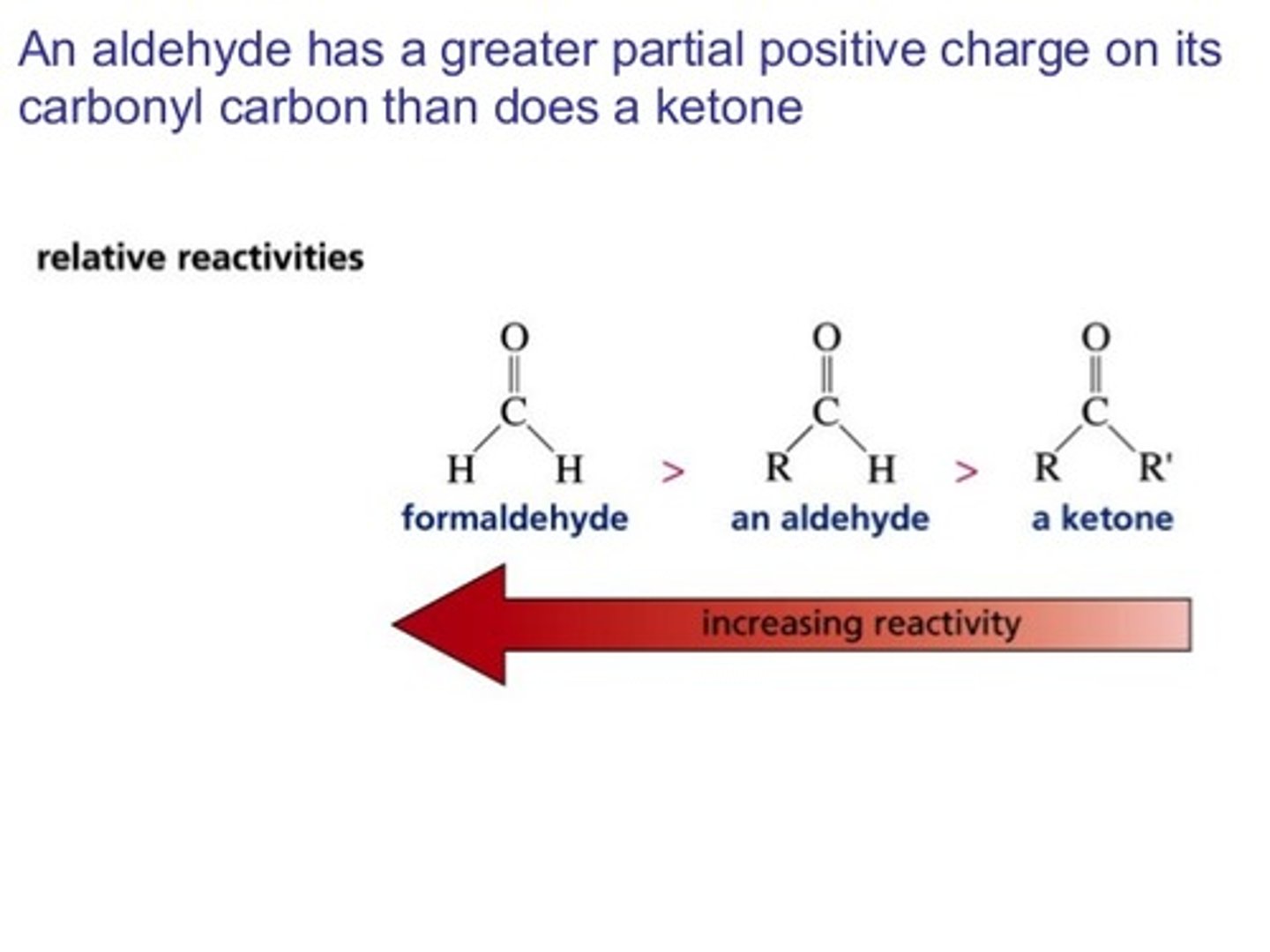
Why do Aldehydes have lower boiling points than Ketones?
The alkyl groups are electron-donating, which will make the carbons have a greater partial-positive charge and the oxgen have a greater partial-negative charge.
True or False? Small ketones and aldehydes are relatively soluble in water.
True. Small ketones and aldehydes are relatively soluble in water. As you increase the chain-length, however, they become less and less soluble.
The oxygen of an aldehyde has what hybridization?
(A) sp3d
(B) sp3
(C) sp2
(D) sp
(C) sp2
The oxygen of an aldehyde has sp2 hybridization.
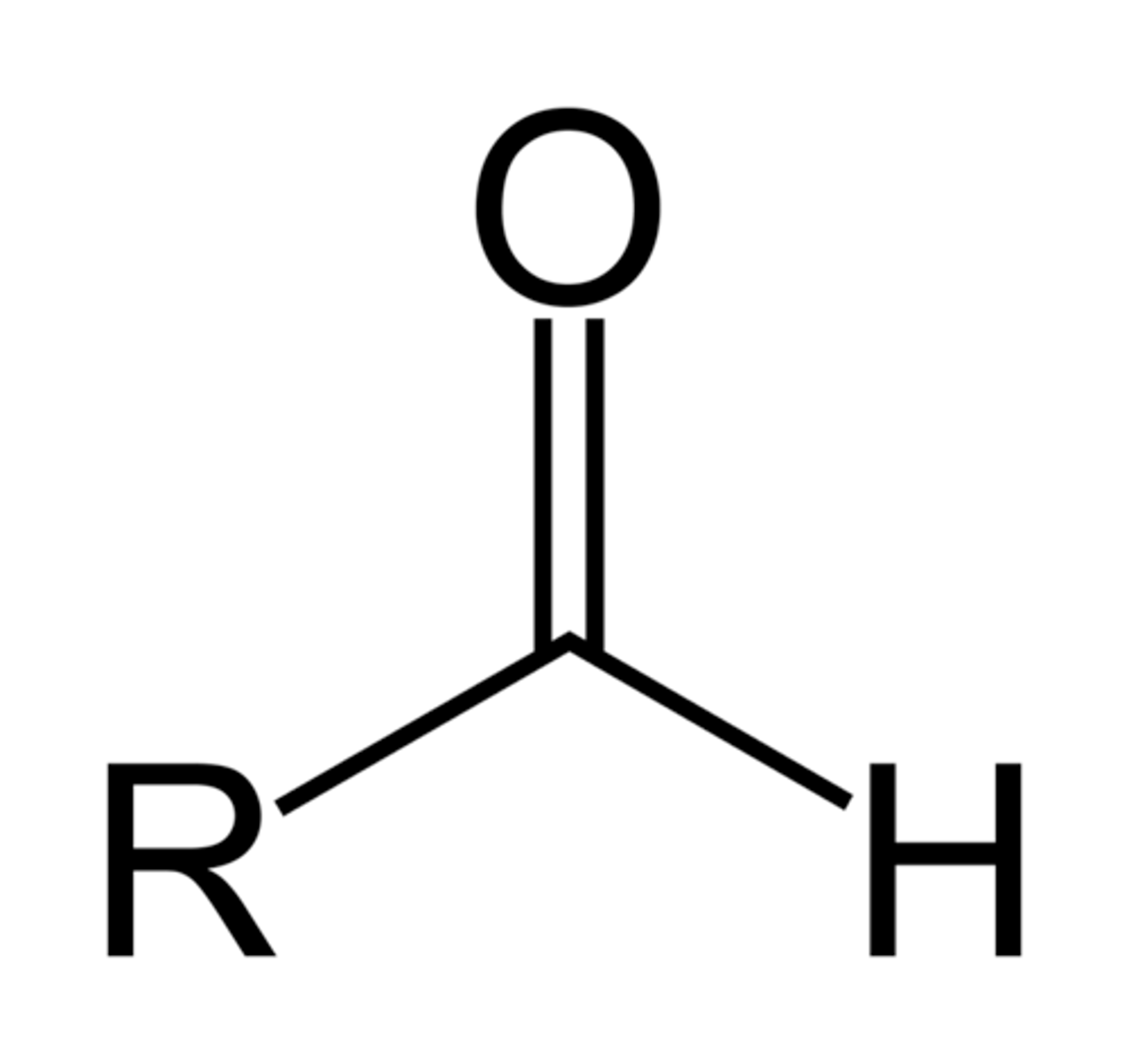
Which are more reactive with nucleophiles? Aldehydes or Ketones? Why (give two reasons)?
Aldehydes are more reactive with nucleophiles than Ketones because Aldehyde has less alkyl groups that donate partial negative charge to the carbonyl carbon. The less negative the carbonyl carbon, the more attractive it is to a nucleophile.
Aldehydes are also less sterically hindered than ketones.
CRB Once a nucleophile has been added to a carbonyl, is there a possibility that the carbonyl is not reformed?
(A) Yes, if there is no good leaving group, the carbonyl will not reform.
(B) Yes, if the carbonyl was an aldehyde and not a ketone, the carbonyl will not reform.
(C) Yes, if the carbonyl was exactly acetone, then the carbonyl will not reform.
(D) No, once a nucleophile has been added to a carbonyl, that carbonyl must be reformed quickly.
(A) Yes, if there is no good leaving group, the carbonyl will not reform.
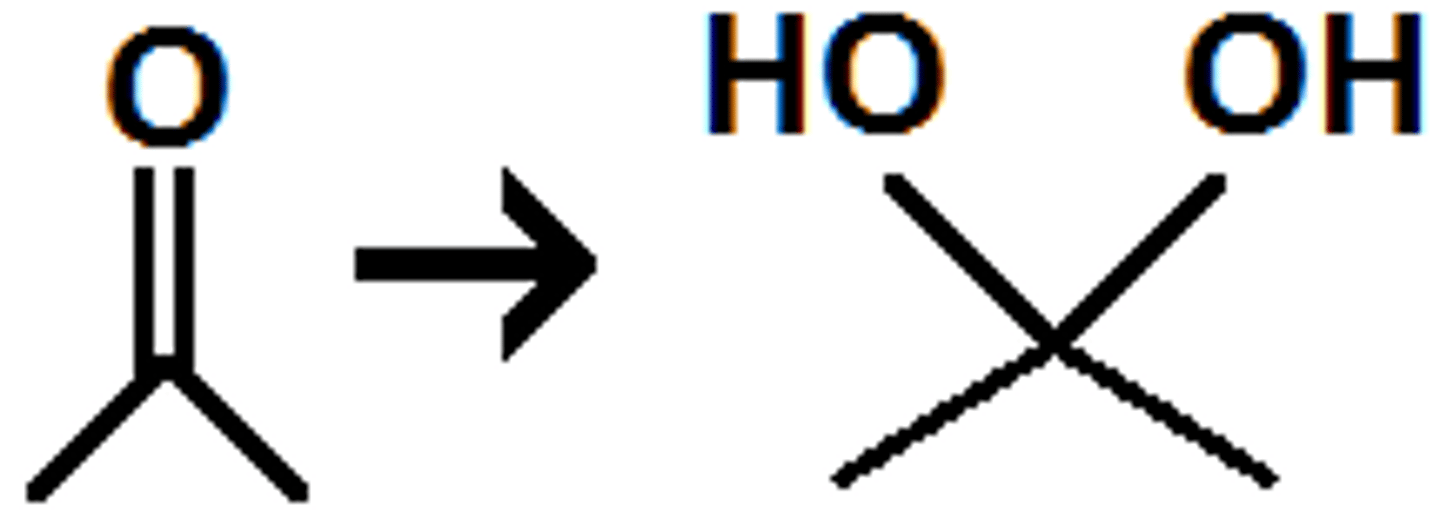
Gem-diols are also termed as:
(A) Ketones
(B) Aldehydes
(C) Hydrates
(D) Alkynes
(C) Hydrates
Gem-diols are also termed as hydrates. This is because they are hydrated ketones/aldehydes.
CRB Functional groups with Nitrogen can be very good nucleophiles, and when added to carbonyls, can form either imines or enamines. Draw the general structure of both imines and enamines.
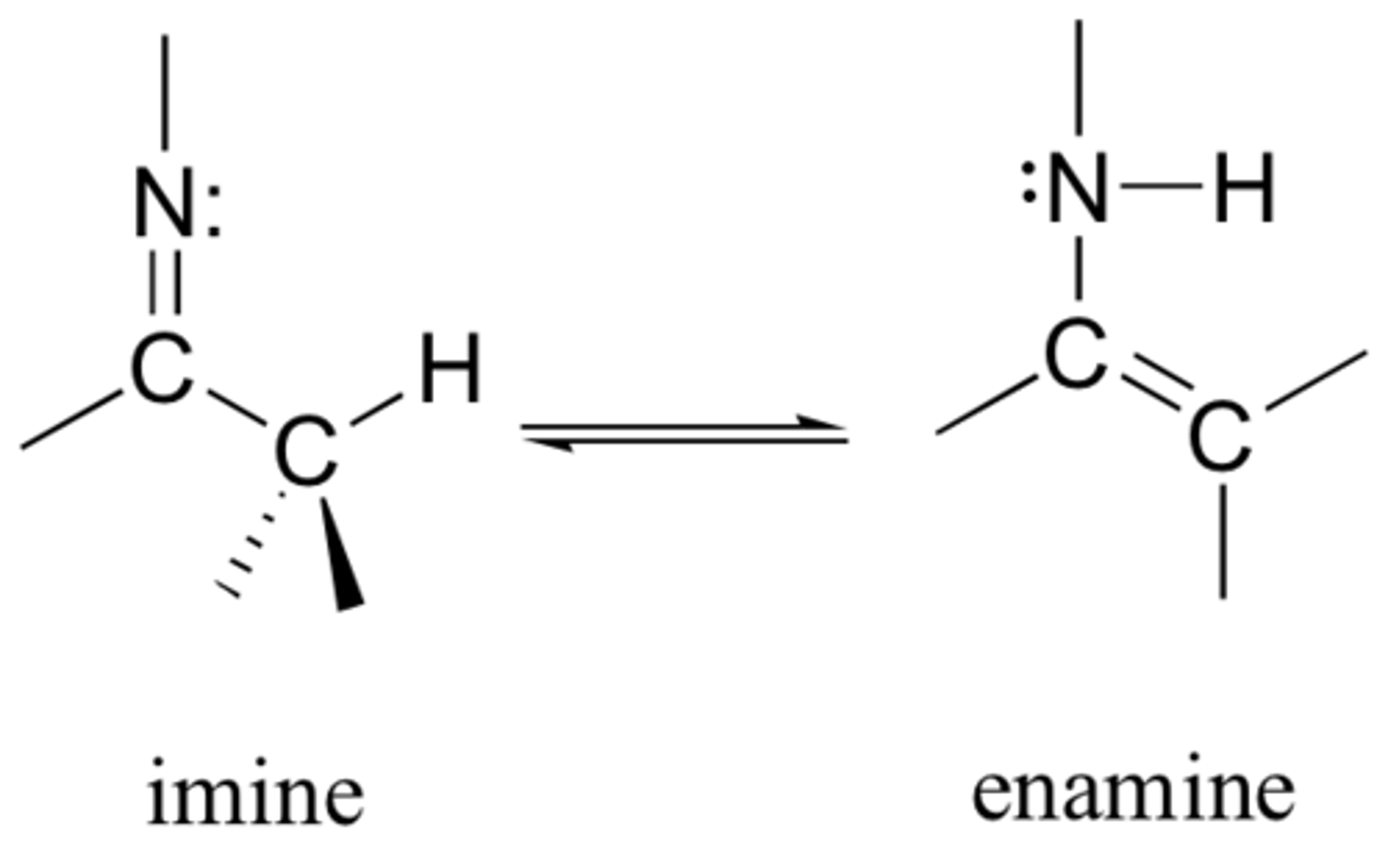
CRB How do the two previous structures relate to the keto and enol forms from the last lesson?
I. Both sets of molecules are constitutional isomers
II. Both are examples of Tautomerizations
III. Both involve Hydrogen Bonding
(A) II only
(B) I and II only
(C) II and III only
(D) I, II and III
(B) I and II only
The relationship between imines/enamines and ketos/enols are that they are:
I. Both sets of molecules are constitutional isomers
II. Both are examples of Tautomerizations
Draw the Hydrate Formation Reaction Mechanism in water.
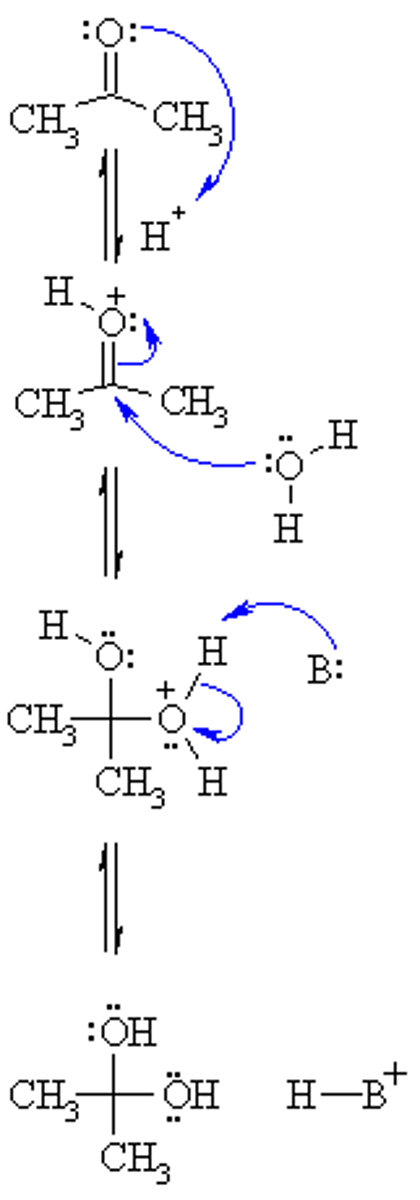
Draw the Hemiacetal Formation Reaction Mechanism in water.
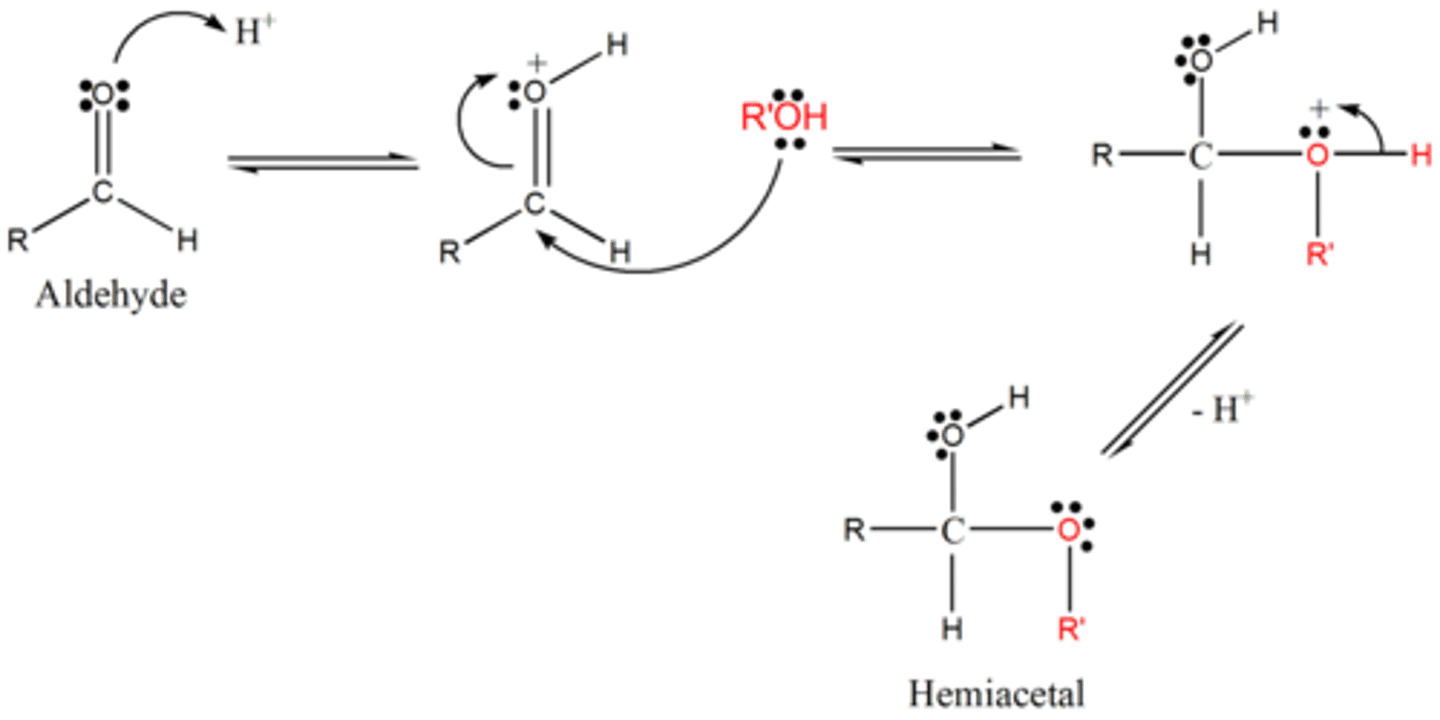
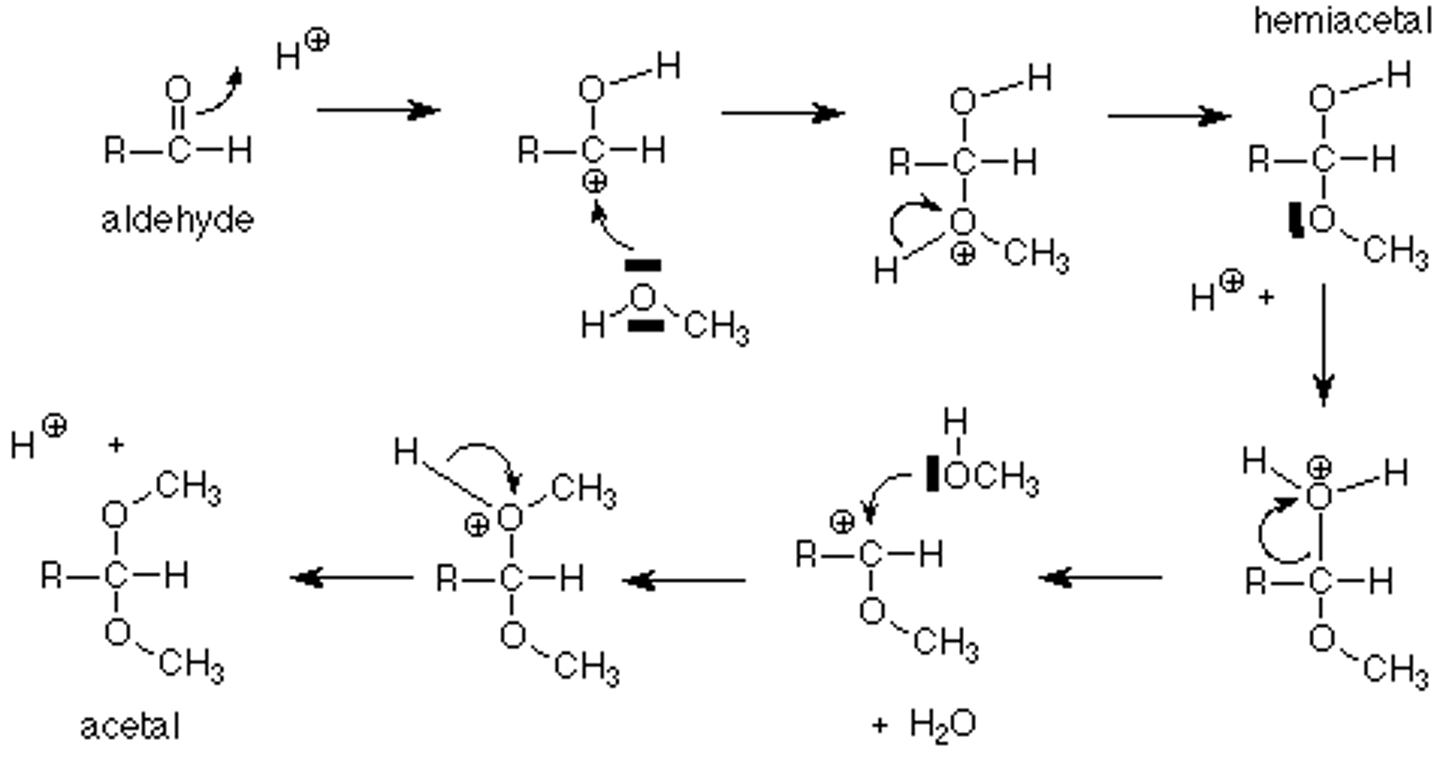
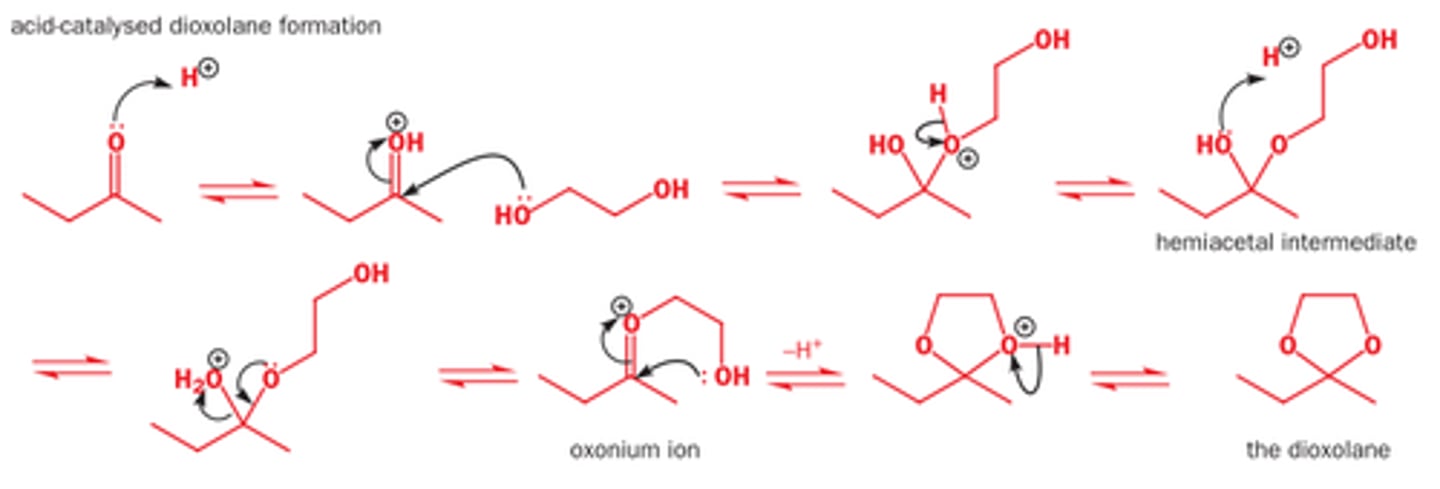
Draw the Hemiacetal and Acetal Formation Reaction Mechanisms in acid.
Draw the Hemiacetal Formation Reaction Mechanisms in base.
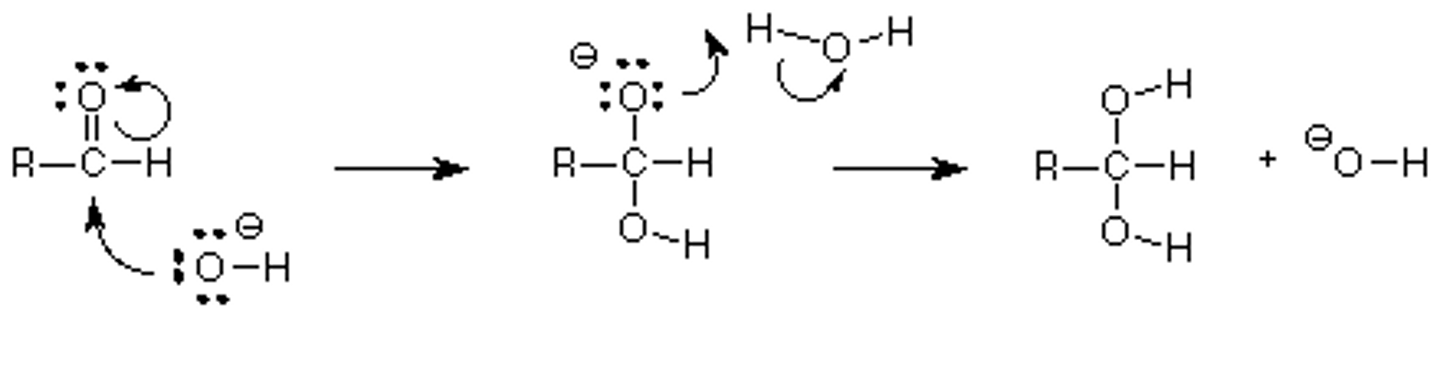
True or False: A hemiacetal cannot be converted to an acetal in acidic conditions, making acetals good protecting group.
False. A hemiacetal cannot be converted to an acetal in BASIC conditions, making acetals a good protecting group.
Which of the following will shift the acetal mechanism to the right toward more product?
I. Add water
II. Increase amount of Aldehyde
III. Increase amount of Alcohol
(A) I Only
(B) I and II Only
(C) II and III Only
(D) I and III Only
(C) II and III Only
The following will shift the acetal mechanism to the right toward more product: REMOVING water (a product), increasing amount of aldehyde (a reactant), increasing amount of alcohol (also a reactant).
Draw the Acetal Formation Mechanism with Ethylene Glycol (ethane 1,2-diol)
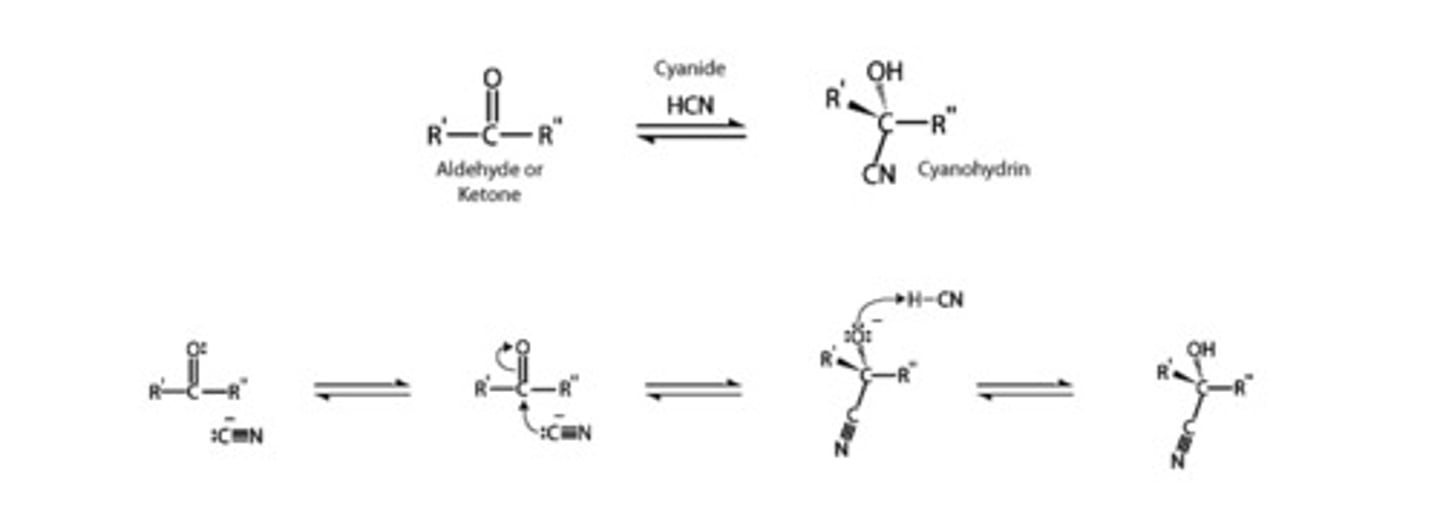
CRB One final key nucleophilic additions to carbonyls is with Cyanide. Draw out the mechanism for creating a cyanohydrin with acid.