Polymers Final
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
polymer solution
polymer is solute (substance being dissolved), some low-molecular weight species are the solvent (doing dissolving)
good solvent
-X < 1/2, B > 0
-solvent molecules separate
B > 0
-good solvent
-monomers on different chains are happy enough to be surrounded by solvent
-when two coils approach to each other, there is steric repulsion (excluded volume) and the chains separate
poor solvent
-Χ > 1/2, B < 0
-polymer chains prefer self interaction
B < 0
-poor solvent
-polymer is barely able to stay dissolve in solution
-monomers find it energetically favorable to be close to other monomers
-when two coils approach each other, there is a tendency to cluster
-all solvent molecules excluded from polymer chain that is coiled, can’t penetrate polymer
theta solvent
-Χ = 1/2, B = 0
-"not-very-good" solvent
-excluded volume and relatively unfavorable solvent-solute interactions cancel one another in the net
-Following Flory-Huggins, it's a θ solvent
interaction parameter (chi - Χ)
-represent exchange energy per molecule normalized by thermal energy kT
-used in enthalpy (H) of mixing for RST
-small Χ or negative allows chains to mix
-lower X → smaller H → more likely to dissolve
solubility parameter (delta - δ)
materials with similar energy of vaporization would have similar intermolecular force, therefore might intend to be soluble with each other
N
-contributes to entropy (S)
-if small, limited contribution
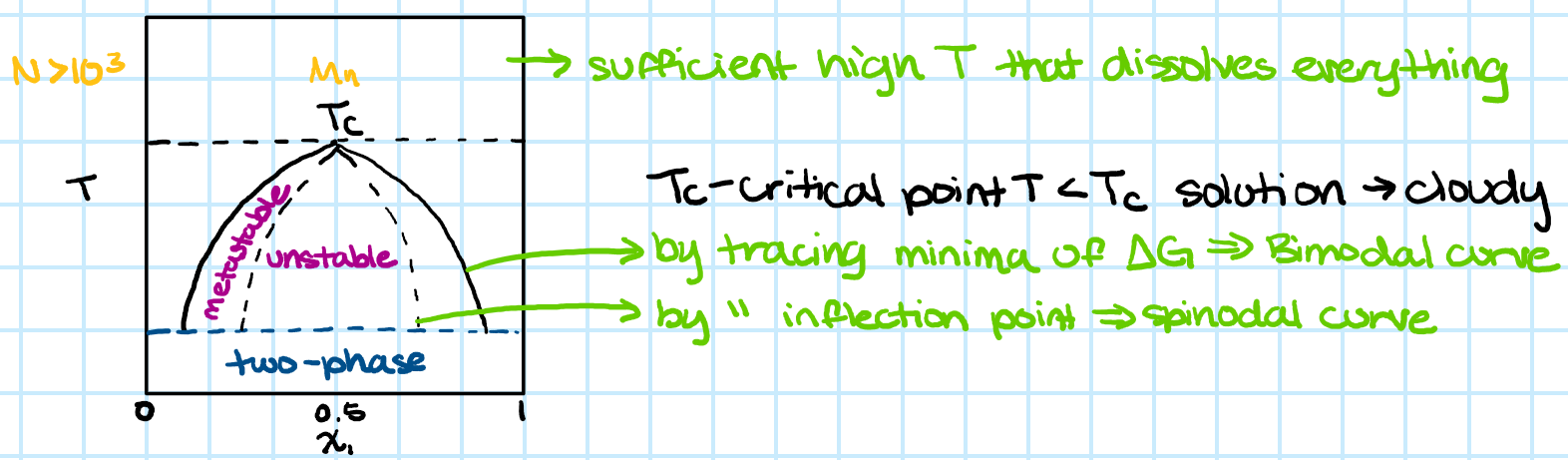
chi N > 10.5
-for block polymer
-indicates polymers will be phase separated
-chi from H (enthalpy) contribution, N from S (entropy) contribution
chi N > 2
-for polymer blends
-indicates polymers will be phase separated
gauche state
preferred configuration for more flexibility because ready to change conformation
molecular weight
-_ must be similar to favor mixing and ensure compatibility in solution
-high _ makes it dissolving of polymer harder
Regular Solution Theory
-description of mixtures of non-polar small molecule liquids with positive or zero mixing enthalpy
-relatively simple statistical model to express free energy of mixing for a binary solution of two
components
regular solution theory where one of the components is a polymer
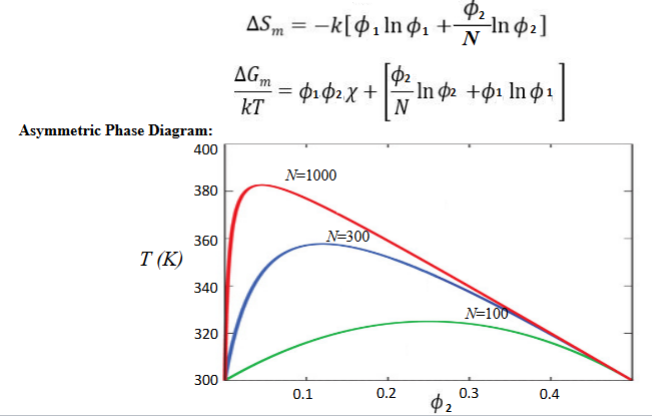
Flory Huggins Theory
ENTROPY (S) of mixing, S > 0 favors mixing
adopt same lattice model as RST but:
-each lattice site has one solvent molecule
-each polymer occupies N lattice sites
-N is proportional to degree of polymerization
-monomer unit was same volume as solvent
uses volume fractions instead of molar fractions (phi, Φ)
polymer-polymer mixtures
-enthalpy contribution does NOT allow for polymer mixing in this case due to N → infinity
-when N → infinity, prevents mixing because S → 0
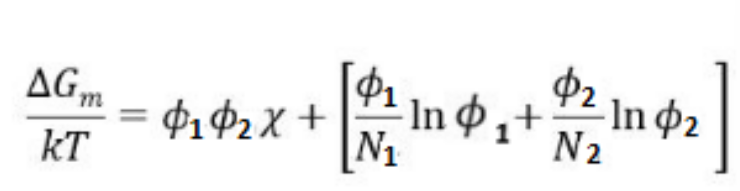
entropy (S)
-a measure of the disorder of a system
-higher _ favors mixing
enthalpy (H)
-total energy/heat of a system
-considers internal change
-high _ opposes spontaneous mixing
-opposes mixing when Χ > 0
-temperature at which an amorphous polymer transitions from a brittle state to a ductile state
-at this temperature, enough energy for polymer chains to slide past each other
-not a thermodynamic property of polymer, can adopt a range of values
factors that affect Tg
-flexibility of polymer backbone (more flexible = lower), means can accommodate to additional change like temperature
-steric effects of pendant groups, more mobility = lower
-configurational isomerism, inefficient packing causes drop
increase free volume
-poly dispersity
-low molecular weight
-plasticizers
-branches
-residual solvent
-inefficient packing
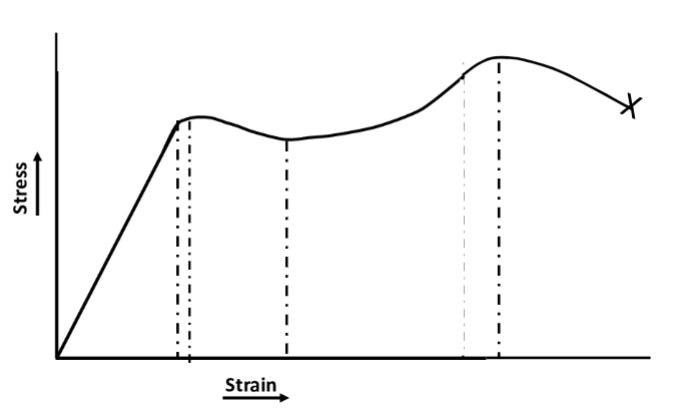
stress vs strain for a semicrystalline polymer
-shows both elastic and plastic deformation regions
-Initially, the material exhibits linear elastic behavior, followed by yielding and eventual necking before fracture.
-amorphous solid→ no long-range order or symmetry in the packing of molecules
-kinetically trapped
-over some range of temperature, molecular motion will become so slow, that an equilibrium packing of molecules can’t be attained during experiment → sample has undergone _
-each polymer has this
-polymer chains locked up in place under Tg, not enough thermal energy to allow chains to slide past, strain prior to fracture is small
-large stresses are dissipated by fracture of covalent bonds, only small strain can be recovered
-smaller stress can be dissipated by local rotation of bonds, sub-Tg relaxation mechanism that contributes to toughness of polymer sample
-glassy polymer still tougher, absorbs more energy prior to fracture than ceramic in general
-changes at Tg less drastic since crystalline regions unaffected
-between Tg and Tm, this polymer is composed of rigid crystallites dispersed in a rubbery amorphous matrix
-at Tm, crystallites melt leading to a viscous state
-heated from low T region, volume expands at constant rate
-at Tg, rate of expansion increases abruptly; change in physical behavior from hard brittle, glassy solid below to a soft rubbery region above
-further heating changes from rubbery to viscous liquid
-becomes stretchy before starts to deform permanently
-entropic elasticity is the dominant restorage force
-~0-10% strain
-stretching a little bit more but slowly, it yields
-breaking the crystalline domain apart and realign them along the strain axis
-suspend it and wait for a day, it will get to the point of ultimate strain
-creep, which is associated with viscoelasticity of polymer samples
-it doesn’t store any mechanical energy, they just dissipate all
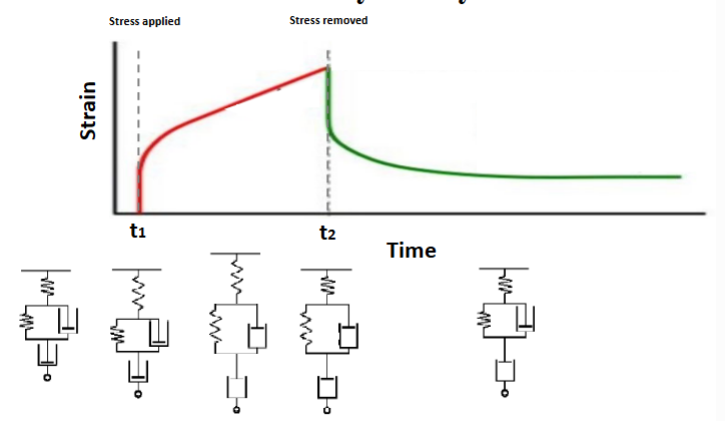
creep
time-dependent deformation at elevated temperature and constant stress
viscosity
-measure of a fluids resistance to flow
-high = thicker fluid, resists motion
-property of materials that exhibit both viscous and elastic characteristics
-when deformed have time-dependent strain behavior that combines elastic recovery with energy dissipation
time-temperature superposition
-a measurement at a certain temperature and time (or frequency) is equivalent to a measurement at a lower temperature and longer time
-experimental time too short → lower temperature to make response long
glassy state
-below Tg
-locked polymer chains
rubber state
-occurs from Tg to Tm
-chain conformations are available
-directly observed shape deformation
sticky fluid
-occurs after Tm
-all polymer chains can slide past each other
-deformation cannot recover
T and S
always positive for dissolving process, system becomes more chaotic after mixing (favors spontaneous mixing)
metal reaction to heat
-heat stretches the metal lattice, causing expansion and changes in properties
-heat enables vibration of molecules → expands
rubber reaction to heat
-rubber is entropic controlled
-stretch out rubber band → heats up
-contract → cools down
unique properties of rubber materials
-entropy (S) driven; in a stretched state, entropic degree of freedom has been reduced
-large deformation
-totally recover after removing stress
creep (for viscoelasticity)
-stress is suddenly applied to a polymer
-resulting strain is a function of time
stress relaxation
-stress is suddenly applied to a polymer
-resulting stress is monitored as a function of time showing dissipating energy
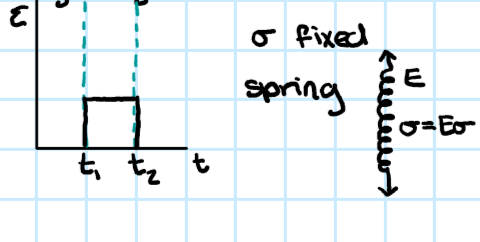
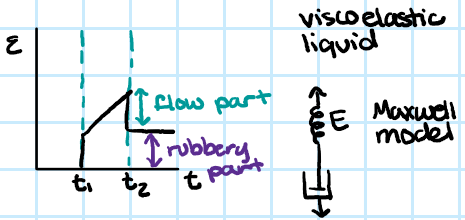
glassy polymer viscoelasticity
-behaves like a real solid
-E > 1 GPa
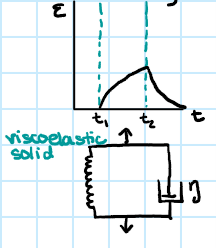
leathery polymer viscoelasticity
-requires time to return to original shape
-time dependence to its elasticity
rubber polymer viscoelasticity
-time dependence partially goes away
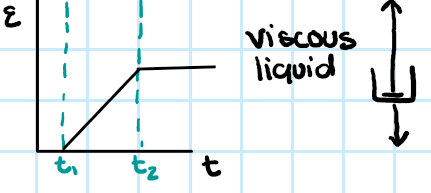
viscous polymer viscoelasticity
polymer molecular mechanism when stretched
-involves chain slippage and realignment under stress, allowing for energy dissipation and recovery
-chain realignment
metal molecular mechanism when stretched
-involves dislocation movement and slip systems, leading to deformation and strain hardening -accumulation of dislocations
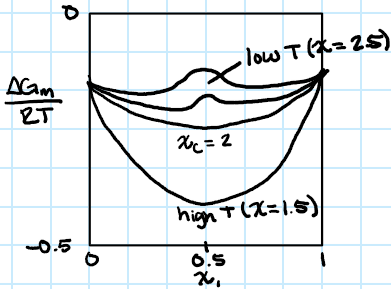
phase diagram (chi vs deltaG/RT)
-chi small → H ignore, G depends on S>0
-chi large → H term produces a local maximum in G which can lead to phase separation or instability in the system
phase diagram (chi vs T)
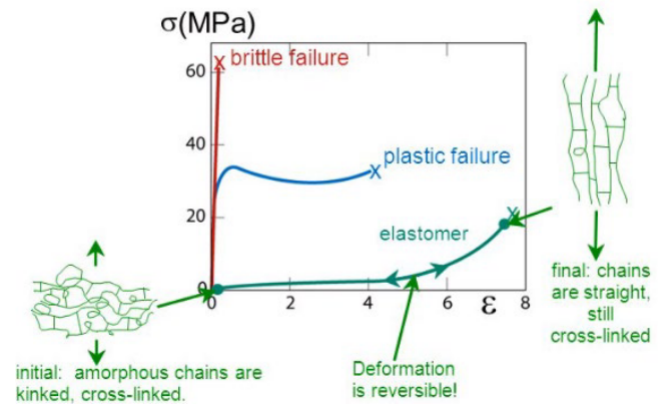
tensile response: elastomer
-no yielding, deformation largely recoverable
-stress increases monotonically up to point of failure
-non-crystalline polymer above Tg has rubber elasticity
-polymer network, gels have rubber elasticity
-rubber elasticity unique to polymeric materials
viscoelasticity of polymers