CHEM 1211 Section 3.6-3.7 (Quantum Numbers and Orbital Shapes)
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
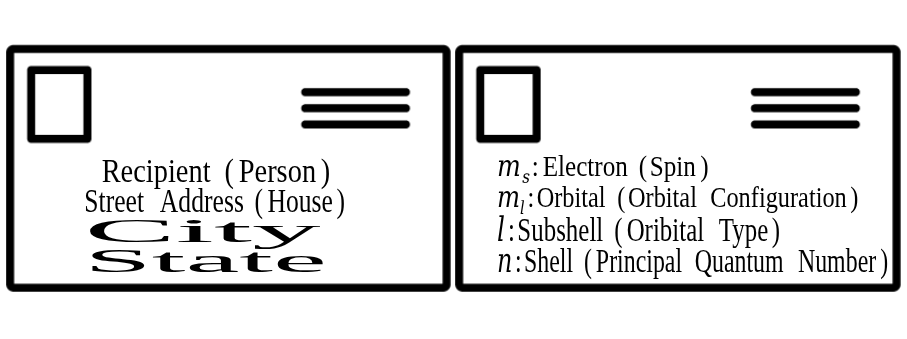
Quantum Numbers as Addresses
A simple way to understand quantum numbers
The Schrodinger Wave Equation
gives a 3D distribution map for the probable position of an electron in an atom
quantum mechanical model
The Schrodinger Wave equation is the basis of the _______ __________ _____ of the atom.
Bohr model
Electrons are not confined to circular orbits as populated by the ____ _____ but can be found nearly anywhere in a spherical space around the nucleus.
greater
The probability of finding an electron in some regions around the nucleus is much _______ than in other regions.
Electron Orbital
A mathematical function predicting how likely it is to find an electron at any given point in space
two electrons
Each Orbital can hold a maximum of ___ _________.
Subshell
a group of orbitals with the same energy
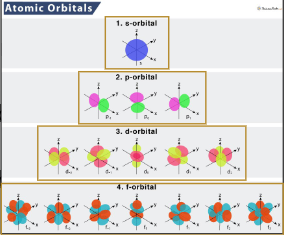
Four Types of Subshells
s, p, d and f
1
Number of orbitals in the s subshell
3
Number of orbitals in the p subshell
5
Number of orbitals in the d subshell
7
Number of orbitals in the f subshell
double
The maximum electron number in a subshell is gonna be ______ the number of orbitals, due to each Orbital having a maximum of two electrons.
Electron Shell (Principal Energy Level [n[)
a grouping of subshells that may be any positive integer