IB Biology HL - Metabolism and Photosynthesis
0.0(0)
Card Sorting
1/25
Earn XP
Description and Tags
Last updated 11:46 AM on 4/26/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
1
New cards
What is cell respiration?
It is the controlled release of energy from organic compounds to produce ATP.
* anaerobic respiration - partial breakdown of glucose in cytosol, no oxygen needed, small yield of ATP
* aerobic respiration - complete breakdown of glucose in mitochondria, larger yield of ATP
* anaerobic respiration - partial breakdown of glucose in cytosol, no oxygen needed, small yield of ATP
* aerobic respiration - complete breakdown of glucose in mitochondria, larger yield of ATP
2
New cards
Why is ATP the source of energy for a cell?
ATP (adenosine triphosphate) contains three covalently linked phosphate groups which store a lot of potential energy in their high-energy bonds. When ATP is hydrolysed to form ADP + Pi, the energy stored in the phosphate bond is released and used by the cell
3
New cards
Outline the process of glycolysis.
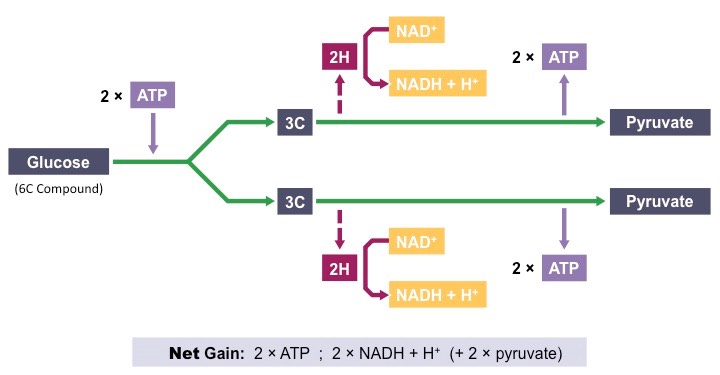
General idea: In the process of glycolysis, glucose (6-C) is broken into two molecules of pyruvate (3-C). Additionally, small yield of ATP (net gain of 2 ATP molecules per 1 glucose) and 2 hydrogen carriers (NADH) from its oxidised precursor NAD+.
1. Phosphorylation
* hexose sugar (typically glucose) is phospholyrated by two molecules of ATP to form a hexose diphosphate
* it makes the molecule less stable and more reactive, as well as prevents its diffusion out of the cell
2. Lysis
* the hexose biphosphate (6-C) is split into two tripse phosphates (3-C)
3. Oxidation
* hydrogen atoms are removed from each of the 3C sugars (via oxidation) to reduce NAD+ to NADH + H+
* two molecules of NADH are produced (one from each 3C sugar)
4. ATP formation
* some energy released from the sugar intermediates is ised to directly synthesize ATP in a process called substrate level phosphorylation
Net gain of glycolysis is: 2 pyruvate molecules, 2 NADH + H+ and 2 ATP molecules
1. Phosphorylation
* hexose sugar (typically glucose) is phospholyrated by two molecules of ATP to form a hexose diphosphate
* it makes the molecule less stable and more reactive, as well as prevents its diffusion out of the cell
2. Lysis
* the hexose biphosphate (6-C) is split into two tripse phosphates (3-C)
3. Oxidation
* hydrogen atoms are removed from each of the 3C sugars (via oxidation) to reduce NAD+ to NADH + H+
* two molecules of NADH are produced (one from each 3C sugar)
4. ATP formation
* some energy released from the sugar intermediates is ised to directly synthesize ATP in a process called substrate level phosphorylation
Net gain of glycolysis is: 2 pyruvate molecules, 2 NADH + H+ and 2 ATP molecules
4
New cards
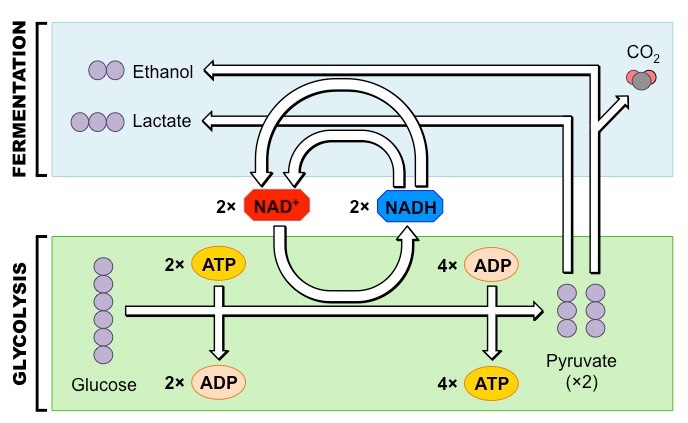
Outline the process of anaerobic respiration.
* if no oxygen is present, pyruvate produced in glycolysis undergoes anaerobic respiration (fermentation). No further ATP is produced
* pyruvate remains in cytosol amd is converted into lactic acid (animals) or ethanol and CO2 (plants, yeast) - this conversion is reversible and is necessary to ensure that glycolysis can continue to produce small quantities of ATP
* it is possible because fermentation restores the stocks of NAD+ necessary for glycolysis
* pyruvate remains in cytosol amd is converted into lactic acid (animals) or ethanol and CO2 (plants, yeast) - this conversion is reversible and is necessary to ensure that glycolysis can continue to produce small quantities of ATP
* it is possible because fermentation restores the stocks of NAD+ necessary for glycolysis
5
New cards
How is anaerobic respiration used to produce bread, ethanol and diary products?
* anaerobic respiration (fermentation) in yeast produces CO2 to elevate the bread dough and alcohol
* bacterial cultures undergo fermentation to produce lactic acid, which modifies milk proteins to generate yogurts and cheeses.
* bacterial cultures undergo fermentation to produce lactic acid, which modifies milk proteins to generate yogurts and cheeses.
6
New cards
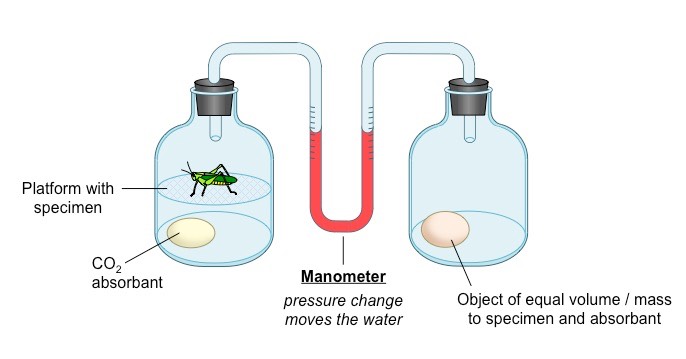
What is a respirometer?
A device that determines the organism’s respiration rate by measuring the rate of exchange of O2 and CO2.
* the living specimen is enclosed in a sealed container
* CO2 production is measured with data logger or by pH changes is the specimen is immersed in water
* when an alkalinis imcluded to absorb CO2, oxygen consumption can be measured as a change in pressure within the system (using a data logger or a U- tube manimeter
* the living specimen is enclosed in a sealed container
* CO2 production is measured with data logger or by pH changes is the specimen is immersed in water
* when an alkalinis imcluded to absorb CO2, oxygen consumption can be measured as a change in pressure within the system (using a data logger or a U- tube manimeter
7
New cards
Define metabolism.
The sum total of all reactions that occur within an organism in order to maintain life.
8
New cards
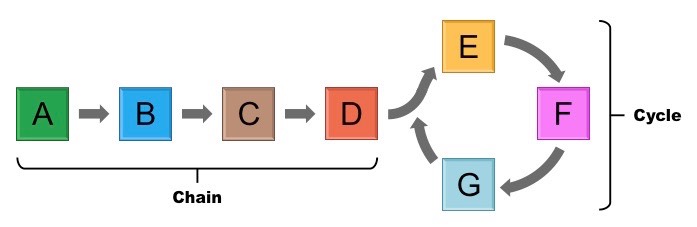
What are metabolic pathways?
Series of reactions contributing to a certain chemical change in a cell. Each of the steps is controlled by a specific enzyme, which allows for a greater level of regulation.
* chain reactions, e.g. glycolysis (in cell resporation), coagulation cascade (in blood clotting)
* cycles, e.g. Krebs cycle (in cell respiration), Calvin cycle (in photosynthesis)
* chain reactions, e.g. glycolysis (in cell resporation), coagulation cascade (in blood clotting)
* cycles, e.g. Krebs cycle (in cell respiration), Calvin cycle (in photosynthesis)
9
New cards
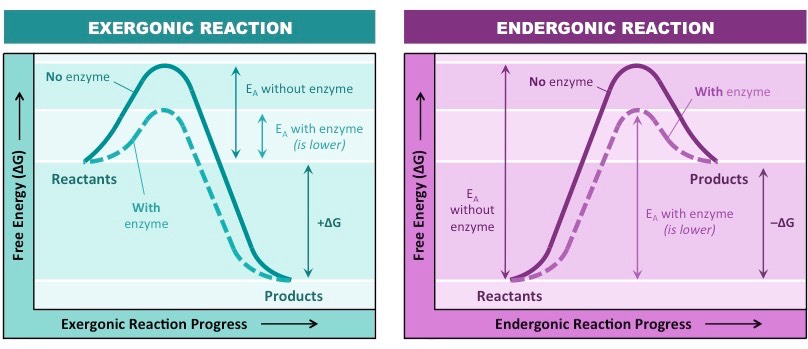
What are the two types of enzymatic reactions?
* exergonic - energy is released from the system, usually catabolic reactions (breakown)
* endergonic - energy is absorbed by the reaction, usually anabolic reactions (building up)
* endergonic - energy is absorbed by the reaction, usually anabolic reactions (building up)
10
New cards
What is an enzyme inhibitor?
A molecule that disrupts the normal reaction pathway between an enzyme and a substrate by preventing the formation of an enzyme-substrate complex and hence, also the formation of product. Can be either competitive or non-competitive.
11
New cards
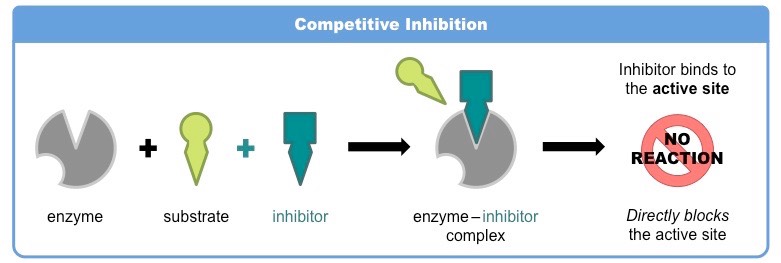
Describe competetive inhibition, including an example.
* a molecule other than the substrate binds to the enzyme’s ==active site==
* this molecule, called an inhibitor, is structurally and chemically similar to the substrate (hence able to bind to the active site)
* the competitive inhibitor blocks the active site and prevents the substrate binding
* as the inhibitor is in competition with the substrate, its effects can be reduced by increasing substrate concentration
Example: ==treatment of influenza via neuraminidase Relenza==
* virions are released from infected cells when the viral enzyme ==neuraminidase== cleaves a docking protein ==haemagglutinin==
* Relenza competitively binds to the neuraminidase active site and prevents the cleavage of the docking protein
* virions are therefore not released from the cell, preventing the spread of the influenza virus
* this molecule, called an inhibitor, is structurally and chemically similar to the substrate (hence able to bind to the active site)
* the competitive inhibitor blocks the active site and prevents the substrate binding
* as the inhibitor is in competition with the substrate, its effects can be reduced by increasing substrate concentration
Example: ==treatment of influenza via neuraminidase Relenza==
* virions are released from infected cells when the viral enzyme ==neuraminidase== cleaves a docking protein ==haemagglutinin==
* Relenza competitively binds to the neuraminidase active site and prevents the cleavage of the docking protein
* virions are therefore not released from the cell, preventing the spread of the influenza virus
12
New cards
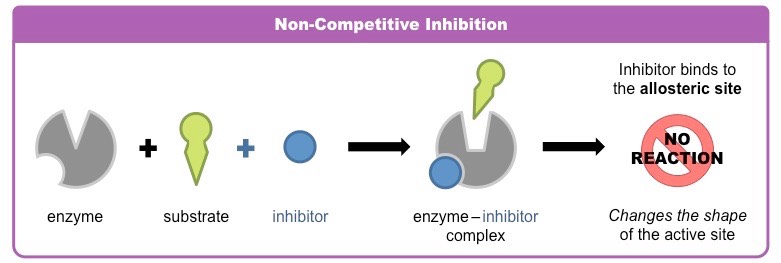
Describe non-competitive inhibition, using an example.
* a molecule binds to a site other than the active site - ==allosteric site==
* the binding of the non-competitive inhibitor to the allosteric side causes a ==conformational change== to the enzyme’s active site
* as a result of this change, the active site and substrate no longer share specificity, so the substrate cannot bind
Example: use of ==cyanide as poison==
* cyanide prevents ATP production via aerobic respiration, leading to death
* it binds to an allosteric site of ==cytochrome oxidase==- a carrier molecule that forms a part of the electron transport chain
* cytochrome oxidase cannot pass electrons to the final acceptor (oxygen) and the ATP is not produced
* the binding of the non-competitive inhibitor to the allosteric side causes a ==conformational change== to the enzyme’s active site
* as a result of this change, the active site and substrate no longer share specificity, so the substrate cannot bind
Example: use of ==cyanide as poison==
* cyanide prevents ATP production via aerobic respiration, leading to death
* it binds to an allosteric site of ==cytochrome oxidase==- a carrier molecule that forms a part of the electron transport chain
* cytochrome oxidase cannot pass electrons to the final acceptor (oxygen) and the ATP is not produced
13
New cards
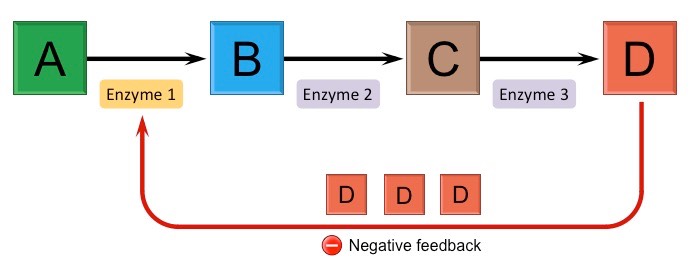
What is an end-product inhibition?
End-product inhibition (feedback inhibition) is a form of negative feedback by which metabollic pathways can be controlled: the final product in a series of reactions inhibits the enzyme from an earlier step in the seauence by binding to its allosteric site a temporarily inactivating it (non-competitive inhibition).
14
New cards
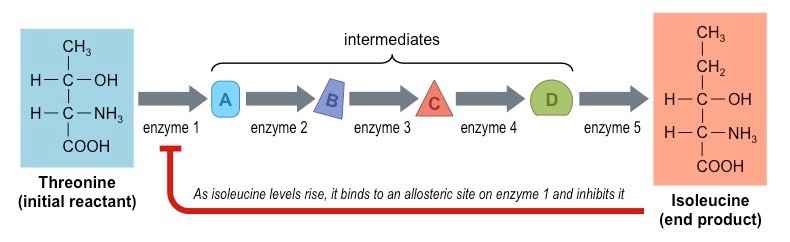
Outline the end-product inhibition of the pathway that converts threonine to isoleucine.
Isoleucine is an essential amino acid (is not synthesized by the body, so must be ingested with food like eggs, seaweed, fish, cheese, chicken, lamb). In plants and bacteria, ==isoleucine== can be synthesized from ==threonine== - this reaction involves 5 steps.
* in the first step, threonine is converted into an intermediate by ==threonine deaminase==
* isoleucine can bind to allosteric site of this enzyme and function as a non-competitive inhibitor
* this ensures that isoleucine production does not cannibalise available stocks of threonine
* in the first step, threonine is converted into an intermediate by ==threonine deaminase==
* isoleucine can bind to allosteric site of this enzyme and function as a non-competitive inhibitor
* this ensures that isoleucine production does not cannibalise available stocks of threonine
15
New cards
What are the two types of electron carriers?
* NAD+ (reduced to NADH)
* FAD (reduced to FADH2)
* FAD (reduced to FADH2)
16
New cards
Describe the process of aerobic respiration.
1. ==Glycolysis== must occur in cytosol to produce ^^pyruvate^^
2. ==Link reaction==
* pyruvate is transported from cytosol into the mitochondrial matrix by carrier proteins on the mitochondrial membrane
* pyruvate loses a carbon atom (^^decarboxylation^^), which forms a CO2
* the 2C compound loses 2 H atoms (this reduces NAD+ to NADH + H+) and becomes an acetyl compound
* this acetyl compound combines with coenzyme A to form ^^acetyl coenzyme A (acetyl CoA)^^
* link reaction occurs twice per glucose molecule (once for each pyruvate)
3. ==Krebs cycle (citric acid cycle/tricarboxylic acid (TCA) cycle)==
* occurs within the matrix of mitochondria
* acetyl CoA transfers its acetyl group to s ^^4-C compound (oxaloacetate)^^ to make a ^^6-C compound (citrate)^^
* over a series, the 6-C compound is broken down to reform the original 4C compound
* this releases 2 carbon atoms via decarboxylation to form two molecules of CO2, produces 3 NADH + H+ and one FADH2, and one molecule of ATP via substrate level phosphorylation
* the cycle occurs twice (once per each CoA), so per one molecule of glucose, the Krebs cycle gives: ^^4CO2, 2 ATP, 6 NADH + H+ and 2 FADH2^^
4. ==Electron transport chain==
* takes place in the ^^inner mitochondrial membrane^^, folded into cristae to increase the SA available for the reaction
* the purpose of the electron transport chain is to release the energy stored in the reduced hydrogen carriers to synthesize ATP
* this is called ^^oxidative phosphorylation^^ (because energy to synthesize ATP is derved from the oxidation of hydrogen carriers)
* steps:
* ^^generating a proton motive force^^ - the hydrogen carriers are oxidised and release high energy electrons and protons. Electrons are transferred to the __electron transport chain__ (several transmembrane carrier proteins) and the energy of the electron movement is used to pump protons into the intermembrane space, creating an ^^electrochemical proton gradient^^
* ^^ATP synthesis via chemiosmosis^^ - as the protons __passively__ move back into the matrix (down the conc. gradien) via transmembrane enzyme ^^ATP synthase^^. It triggers the ^^molecular rotation^^ of this enzyme, synthesizing ATP
* ^^reduction of oxygen^^ - removal of de-energised electrons. Oxygen acts as the ^^final electron acceptor^^ and also binds with the __free protons in the matrix__ to produce ^^water^^
17
New cards
How many molecules of ATP is produced from one glucose in aerobic resporation?
36
18
New cards
What is photosynthesis?
A process by which cells containing a photosynthetic pigment synthesize organic compounds from inorganic molecules in the presence of sunlight
19
New cards
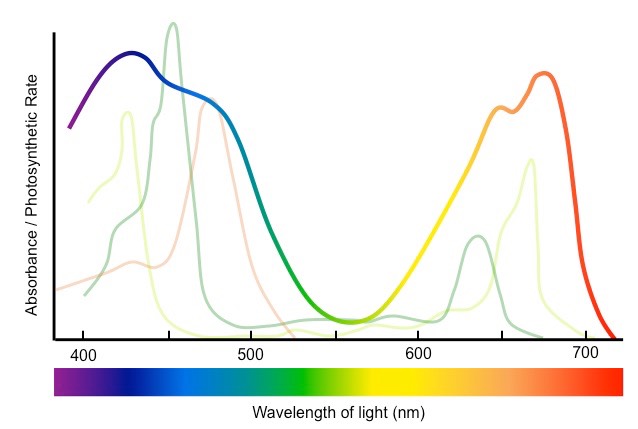
What colours are absorbed and what colours are reflected by chlorophyll?
absorbs mostly blue and red loght
reflects mostly green light
reflects mostly green light
20
New cards
What is absorbtion spectrum and action spectrum of pigments?
Absorbtion spectrum indicates the wavelength of light absorbed by each pigment.
Action soectrum indicates the overall rate of photosynthesis at each wavelength ov light
Action soectrum indicates the overall rate of photosynthesis at each wavelength ov light
21
New cards
What is chromatography?
Experimental technique by which mixtures can be separated.
* a mixture is dissolved in fluid (mobile phase) and passed through a static material, such as paper or silica gel (stationary phase)
* the different components of the mixture travel at different speeds, which causes them to separate
* a retardation factor can be calculated:
Rf = dictance component travels/distance solvent travels
* a mixture is dissolved in fluid (mobile phase) and passed through a static material, such as paper or silica gel (stationary phase)
* the different components of the mixture travel at different speeds, which causes them to separate
* a retardation factor can be calculated:
Rf = dictance component travels/distance solvent travels
22
New cards
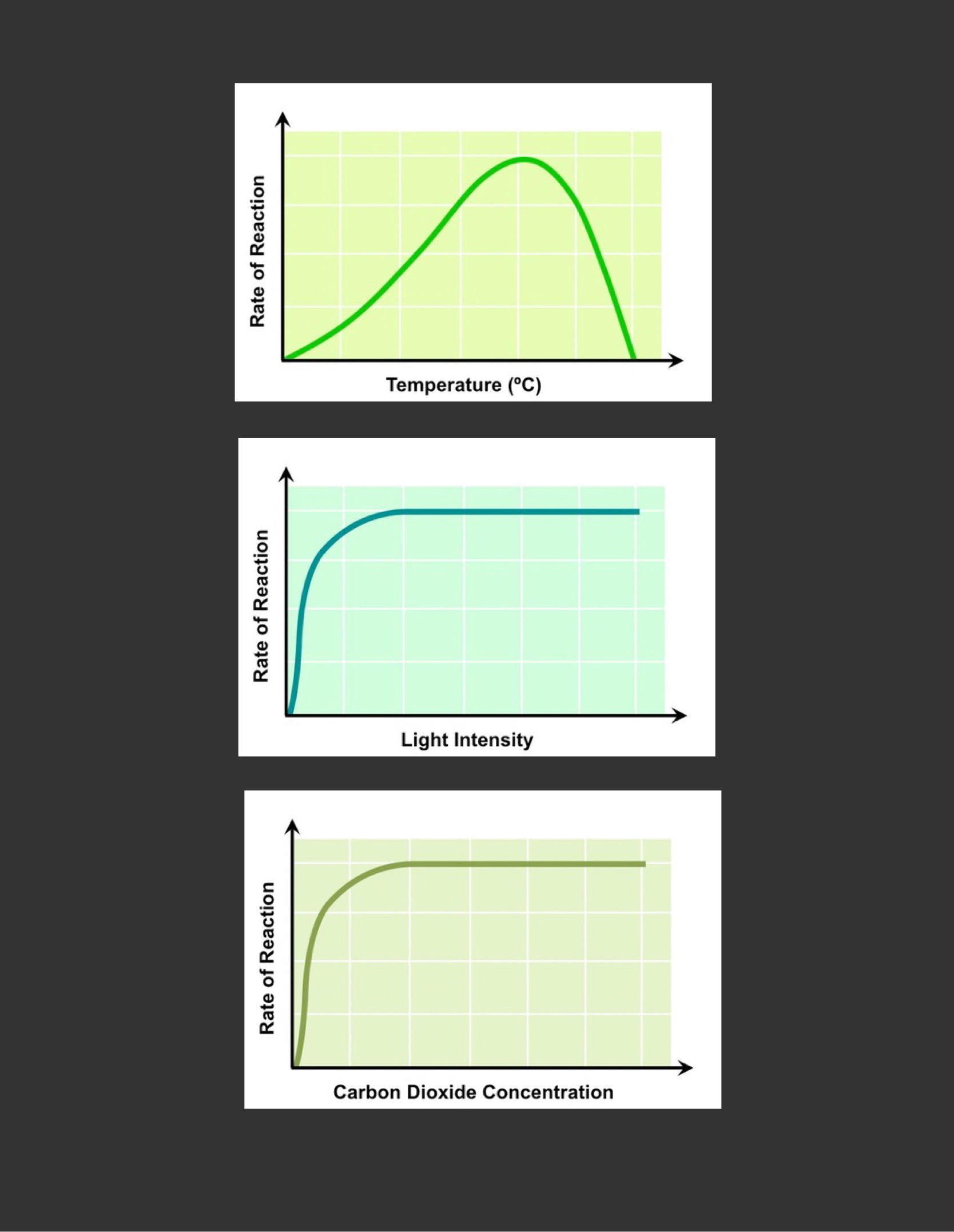
Outline the limiting factors for the rate of photosynthesis.
1. ==Temperature==
* photosynthesis is controlled by enzymes that are senssitive to temperature fluctuations
* as the temperature increases, the rate will be increasing as reactants will have greater kinetic energy and more collisions will result
* above a certain temperature the rate will decrease, as essential enzymes will begin to degenerate
2. ==Light intensity==
* light is absorbed by chlorophyll and as its intensity increases, more and more chlorophyll will be photo-activated
* at certain intensity the rate will plateao, as all available chlorophyll are saturated with light
* different wavelengths of light will have different effects on the rate of photosynthesis
3. ==CO2 concentration==
* it is involved in the fixation of carbon atoms to form organic molecules
* as its concentrations increase, the rate will increase as more molecules are being produced
* at certain concentration of CO2 the rate will plateau, as enzymes responsible for carbon fixation are saturated
23
New cards
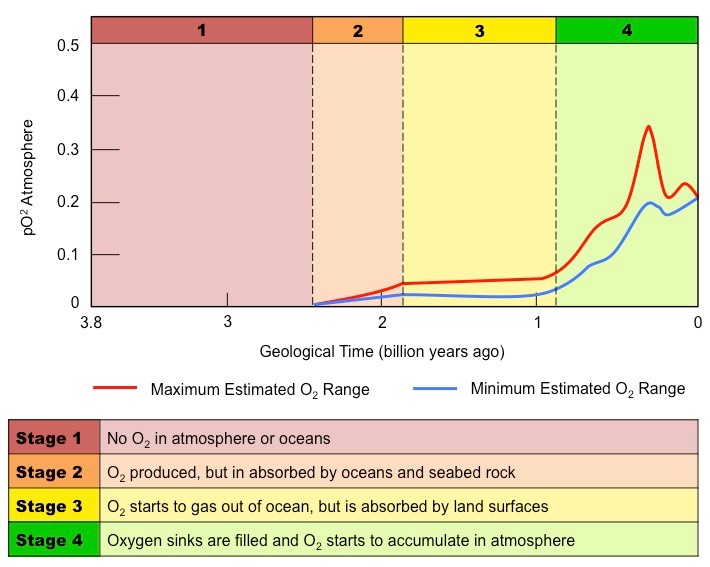
What were/are the effects of oxygenation of Earth?
1. Oceans:
* Earth’s oceans initially had high levels of dissolved iron, released from the crust by underwater volcanic vents
* iron reacted with O2 produced by biological photosynthesis to form insoluble iron oxide
* this process consumed ocean’s iton and the oxygen started accumulating in the atmosphere
2. Atmosphere
* for the first 2 billion years after formation, Earth had anoxic atmosphere
* currently, conc. of oxygen in the atmosphere is \~20%
3. Rock deposition
* the oceanic deposits of iron oxide are called banded iron formations (BIFs) and they are mostly found in oceanic sedimentary rock older than 1.8 billion years old (which gives us information about the time when oxygen levels caused near complete consumption of dissolved iron levels)
* as BIF deposition slowed in oceans, iron rich layers started to form in land due to the rise in O2 levels in atmosphere
4. Biological life
* free oxygen is toxic to obligate anaerobes and increase in O2 may have wiped out many of them
* conversely, it allowed for the evolution of aerobically respiring organisms
24
New cards
Describe the process of photosynthesis.
1. ==Light-dependent reactions== (thylakoid)
* ==excitation of photosystems by light energy==
* **photosystems** are groups of photosynthetic pigments (including chlorophyll) embedded within the thylakoid membrane and are classified according to their max. absorbtion wavelengths (PS I = 700nm, PS II = 680nm)
* when a photosystem absorbs light energy, delocalized electrons within the pigments become **excited**
* the excited electronsare transferred to carrier molecules within the thylakoid membrane
* ==production of ATP via electron transport chain==
* excited electrons from photosystem II (P680) are transferred to an electron transport chain within the thylakoid membrane
* as the electrons are passed through the chain, they lose energy, which is used to **translocate H+ ions** into thylakoid
* this creates the electrochemical gradient (proton motive force)
* H+ ions return tonstroma along the proton gradient via **ATP synthase** (__chemiosmosis__) and ATP is synthesized from ADP + Pi - this process is called ==photophosphorylation==, as light provided the initial energy source for ATP production
* the de-energized electrons are then taken up by the photosystem I
* ==reduction of NADP+ and photolysis of water==
* excited electrons from photosystem I may be transferred to a carrier molecule and used to reduce NADP+ to NADPH (which, together with ATP, is needed in light-independent reactions)
* the electrons lost from photosystem I are replaced by de-energized electrons from photosystem II, and the electrons lost from photosystem II are replaced by electrons released from water via photolysis (water is split by light energy into H+, electrons and oxygen by-product)
2. ==Light-independent reactions (Calvin cycle)== (stroma)
* ==carbon fixation==
* the Calvin cycle begins with a 5-C compound called **ribulose bisphosphate** (**RuBP**)
* an enzyme **RuBP carboxylase** (**Rubisco**) catalyses the attachment of a CO2 molecule to RUBP
* it results in the formation of an unstable 6-C compound which breaks down into two 3-C compounds of **glycerate-3-phosphate (GP)**
* a single cycle involves 3RuBP combining with 3CO2 to make 6GP
* ==reduction of glycerate-3-phosphate==
* GP is converted to **triose phosphate (TP)** using NADPH and ATP - reduction by NADPH transfers hydrogen atoms to the compound, while ATP hydrolysis provides energy
* each GP reauires one NADPH and one ATP to form a triose phosphate
* ==regeneration of RuBP==
* of the six molecules of TP produced per cycle, __one__ may be used to form half a sugar molecule and remaining __5__ are recombined to regenerate RuBP stocks (energy from ATP hydrolysis is required)
25
New cards
Outline the lollipop experiment.
* radioactive carbon-14 is added to a “lollipop” apparatus containing green algae (Chlorella)
* light is shone on the apparatus to induce photosynthesis, which will incorporatevcarbon-14 into organic compounds
* after different periods of time, the algae is killed by running itbinto a solution of heated alcohol (this stops cell metabolism)
* dead algal samples are analysed using 2D chromatography, which separated out the different carbon compounds
* any radioactive carbon compounds on the chromatogram are identified using autorafiography (X-ray film exposure)
* by comparing differentbperiods of light exposure, the order by which cstbon compounds are generated is determined
* light is shone on the apparatus to induce photosynthesis, which will incorporatevcarbon-14 into organic compounds
* after different periods of time, the algae is killed by running itbinto a solution of heated alcohol (this stops cell metabolism)
* dead algal samples are analysed using 2D chromatography, which separated out the different carbon compounds
* any radioactive carbon compounds on the chromatogram are identified using autorafiography (X-ray film exposure)
* by comparing differentbperiods of light exposure, the order by which cstbon compounds are generated is determined
26
New cards
Distinguish between cyclic and non-cyclic photophosphorylation.
1. Cyclic photophosphorylation
* uses only one photosystem (PSI)
* does not involve the reduction of NADP+
* light is absorbed by PSI and the excited electron enters the electron transport chain to produce ATP. When the electron becomes de-energised, it returns to the photosystem, restoringbitselectron supply
* as the electron returns to the photosystem, NaDP+ is not reduced and water is not needed to replenish the elctron supply
2. Non-cyclic photophosphorylation
* involves both photosystem
* requires the reduction of NADP+
* when light is absorbed by PSII , the excited electrons enter the electron transport chain to produce ATP
* concurrently, photoactivation of PSI results in the release of electrons which reduce NAD+ to NaDPH
* the photolysis of water releases electrons which replace those lost by PII
Cyclic photophosphorylation can be used to produce a steady supply of ATP in the presence of sunlight. However, ATP is highly reactive and cannot be stored in the cell and cyclic photophosphorylation does not produce NADPH needed in light-independent reactions. Only non-cyclic photophosphorylation allows for the synthesis of organic molecules and long-term energy storage.