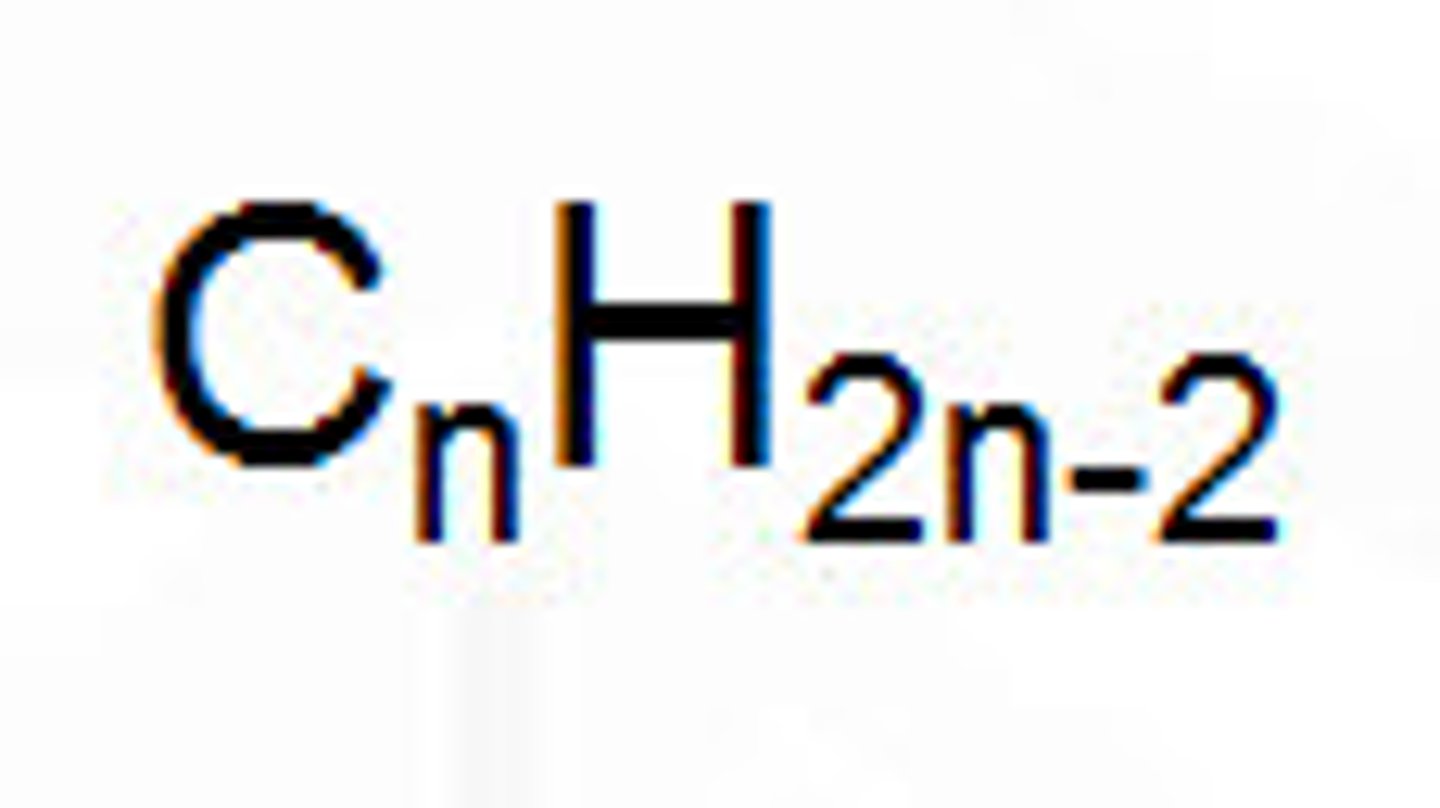Hydrocarbons
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Hydrocarbons
Compounds composed of only carbon and hydrogen
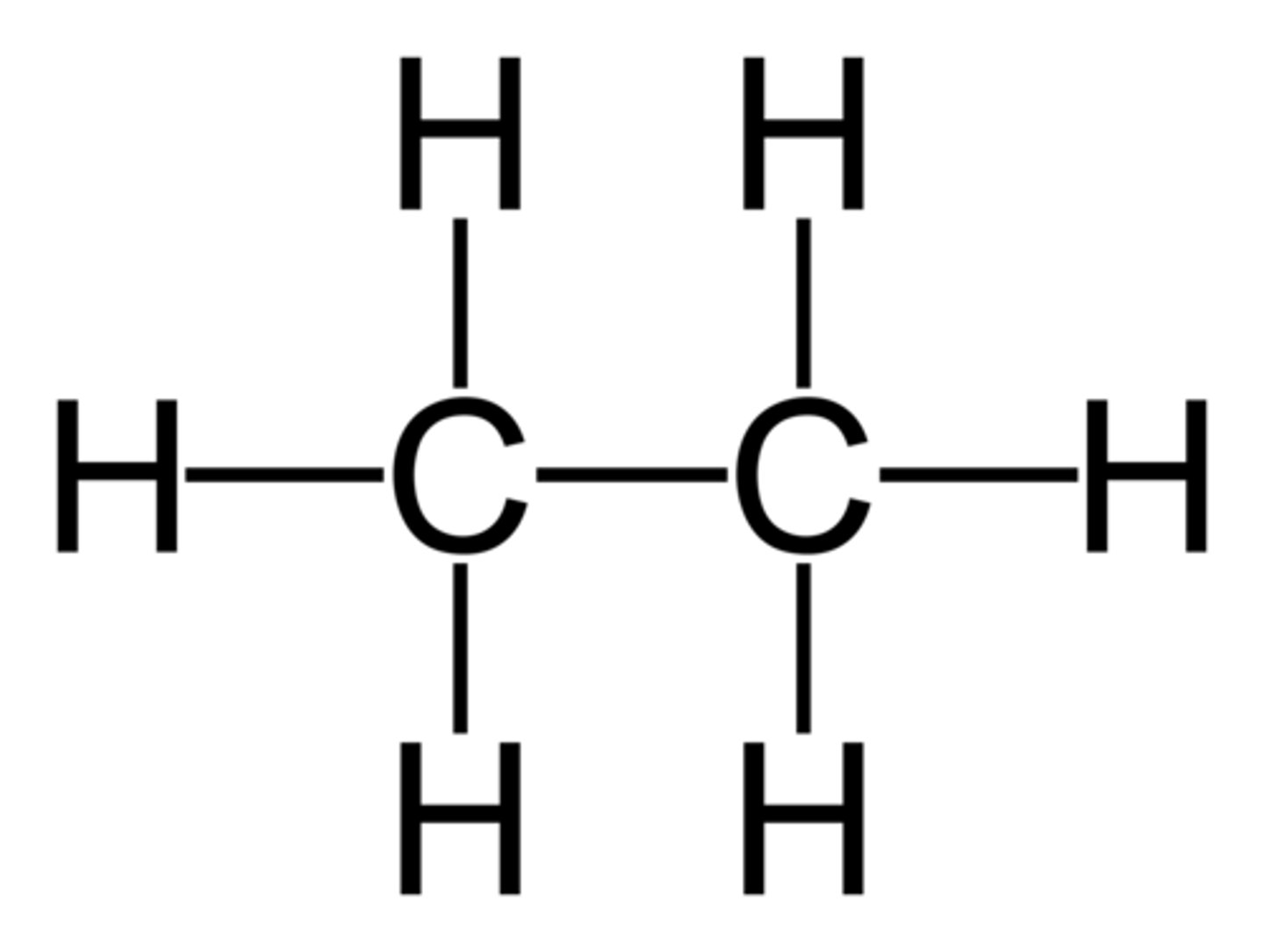
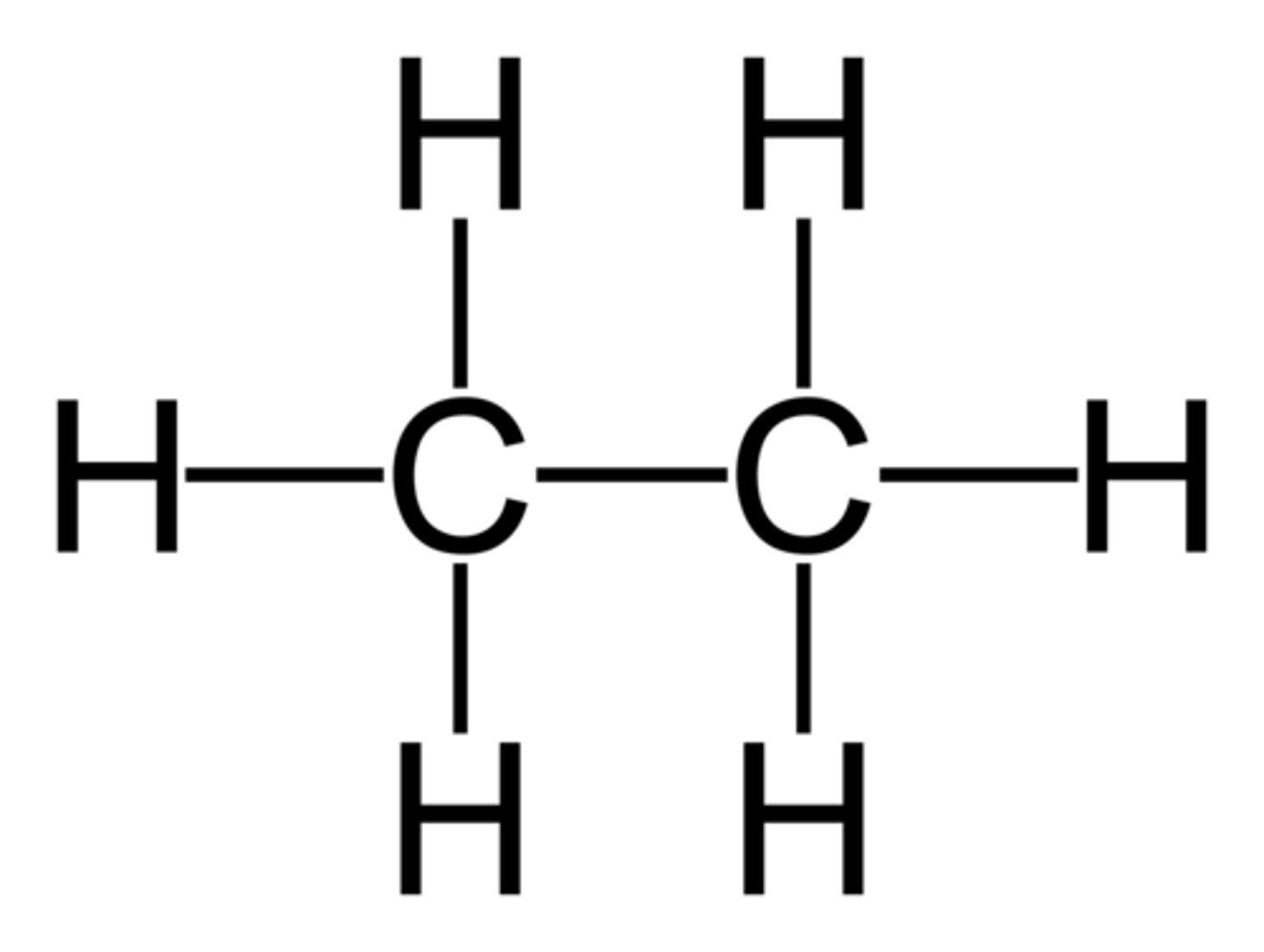
saturated hydrocarbon
a hydrocarbon in which all of the bonds are single bonds
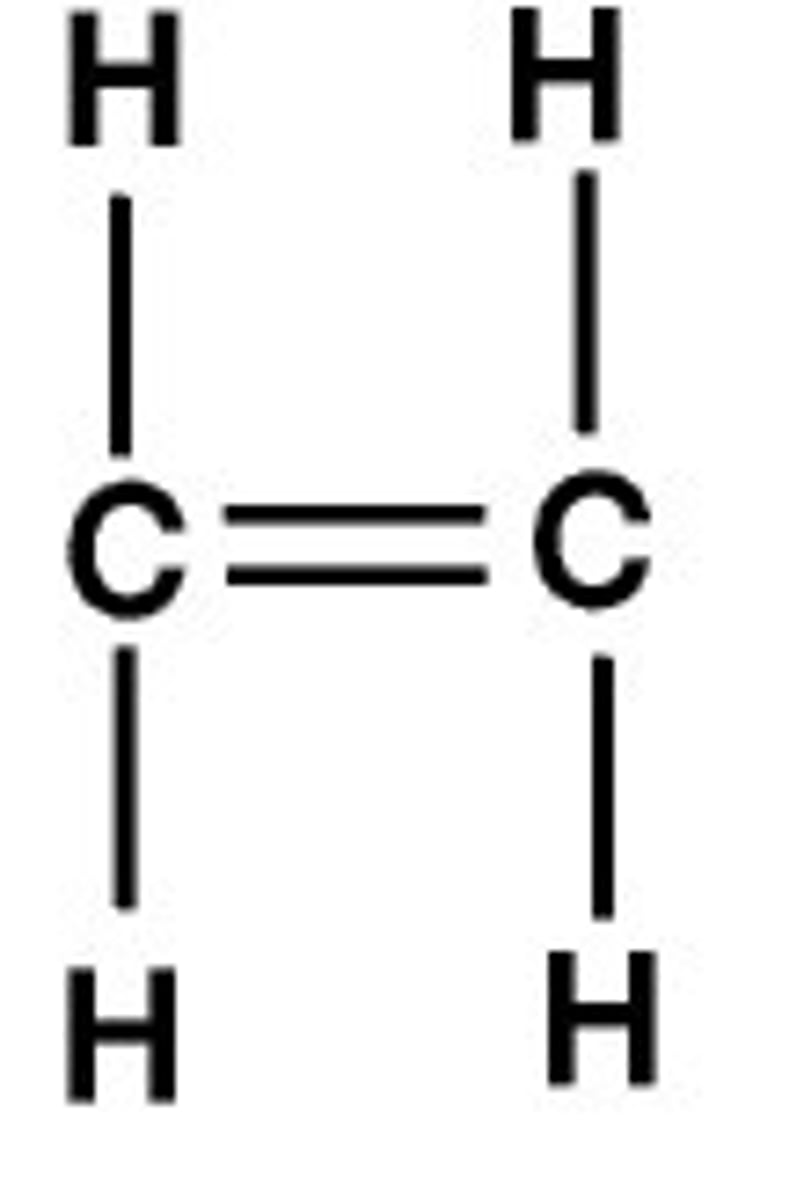
unsaturated hydrocarbon
a hydrocarbon that contains one or more double or triple bonds
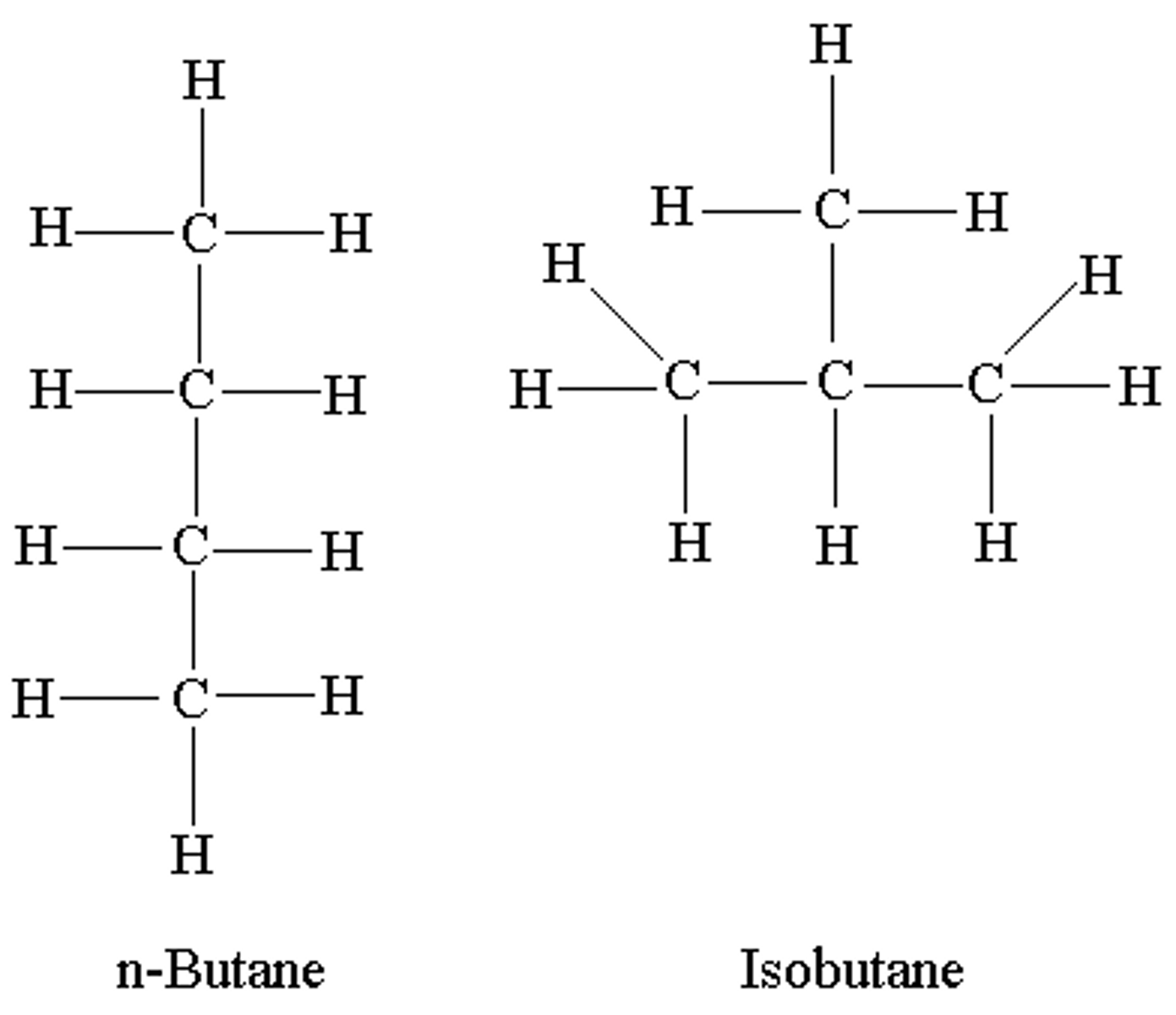
Isomers
Same atoms but different arrangement.
chemical formula
Symbols that show the elements in a compound and the ratio of atoms
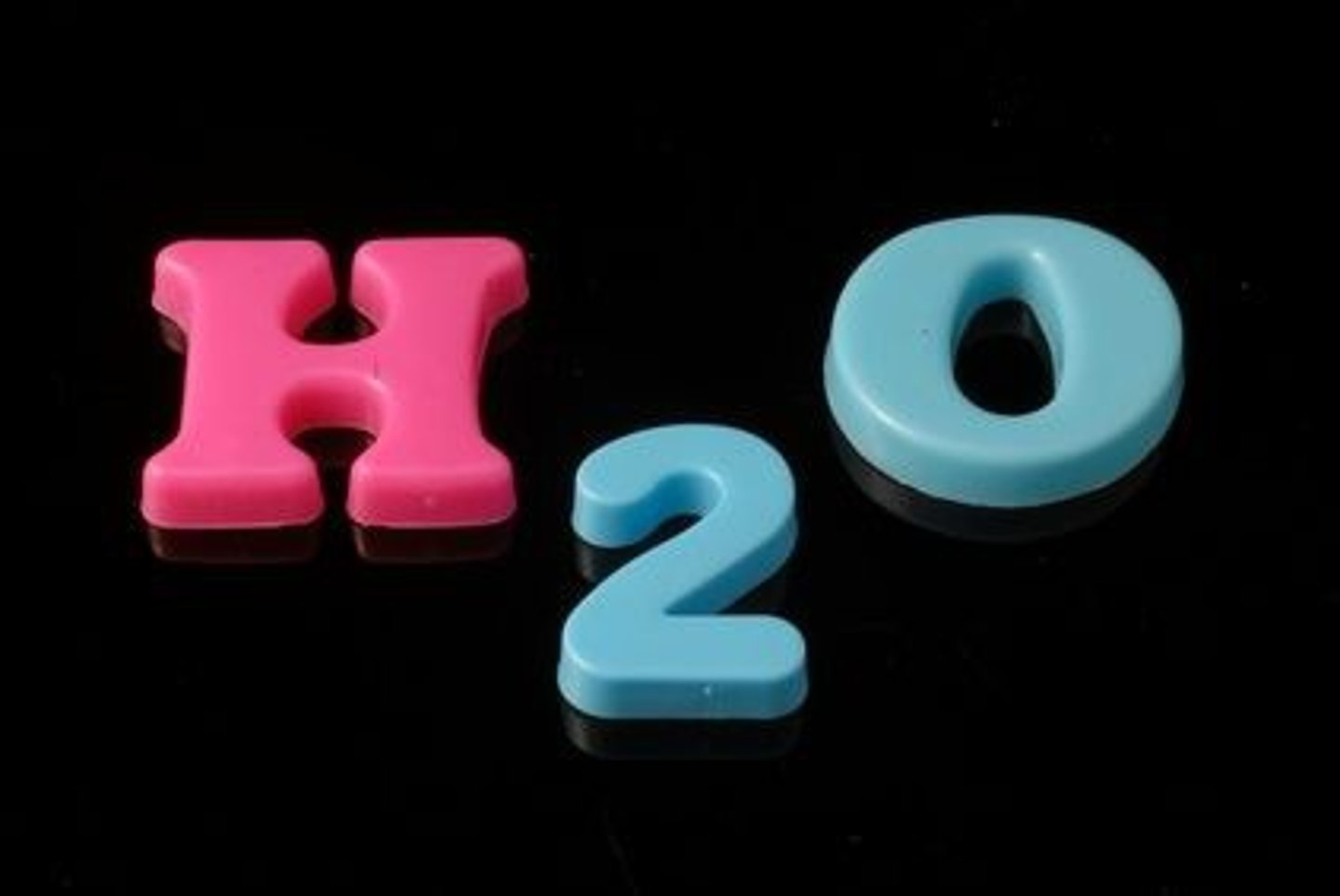
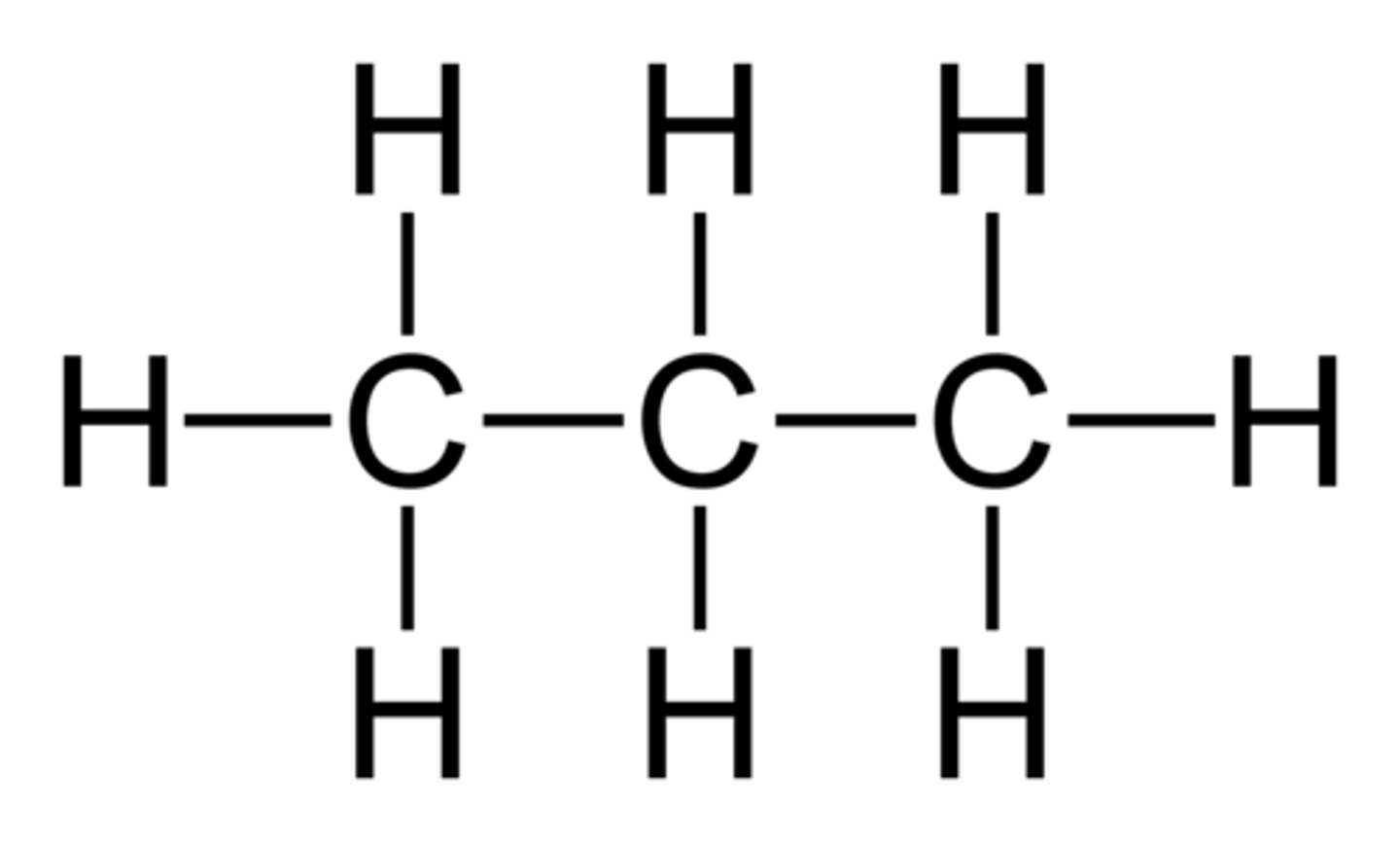
structural formula
a formula that shows the arrangement of atoms in the molecule of a compound (without showing lone pairs of electrons)
Alkane
a hydrocarbon containing only single covalent bonds
Alkene
a hydrocarbon containing a double bond between two carbon atoms
Alkyne
a hydrocarbon containing a triple bond between two carbon atoms
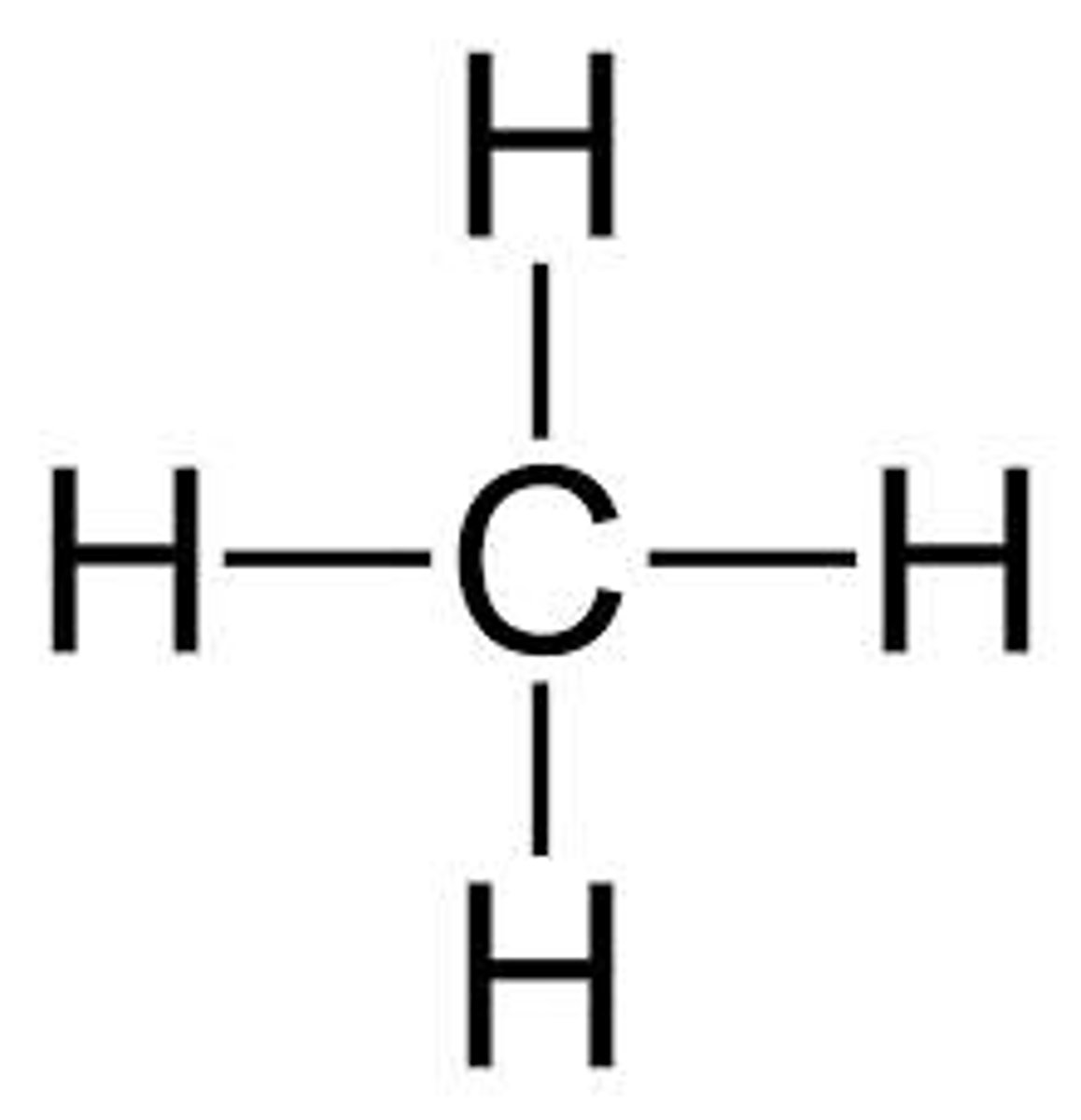
Methane
CH4
Ethane
C2H6
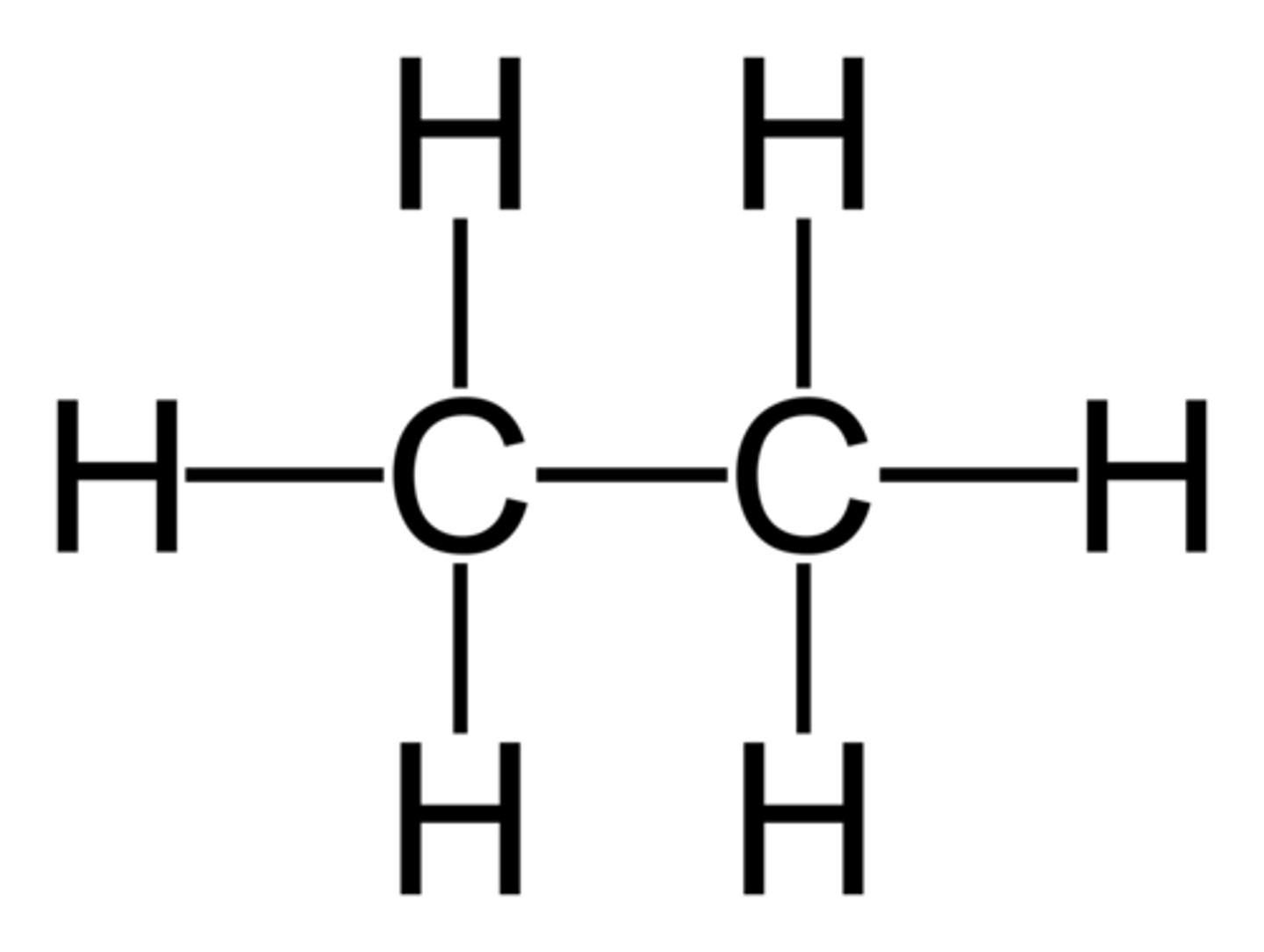
Propane
C3H8
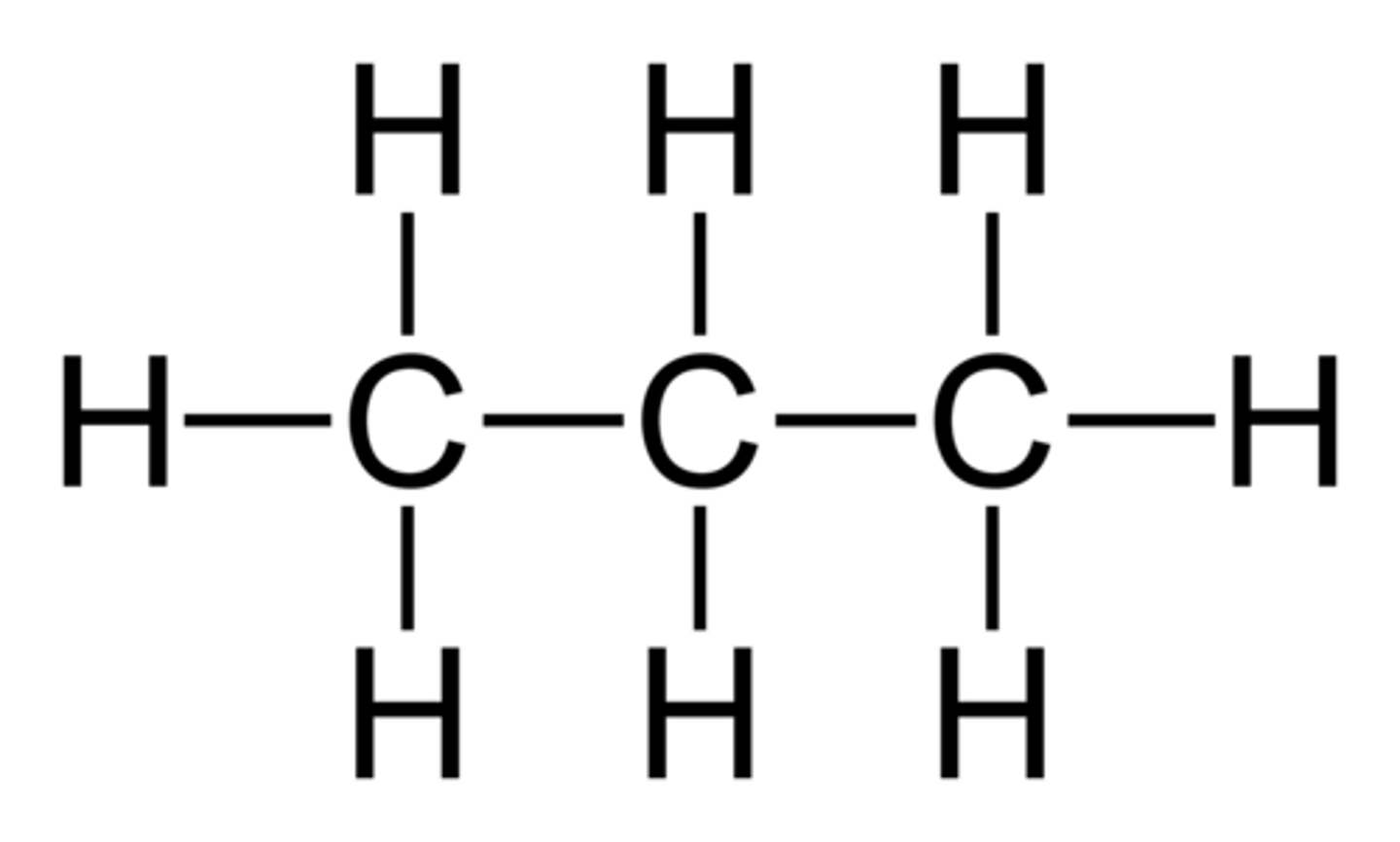
Butane
C4H10
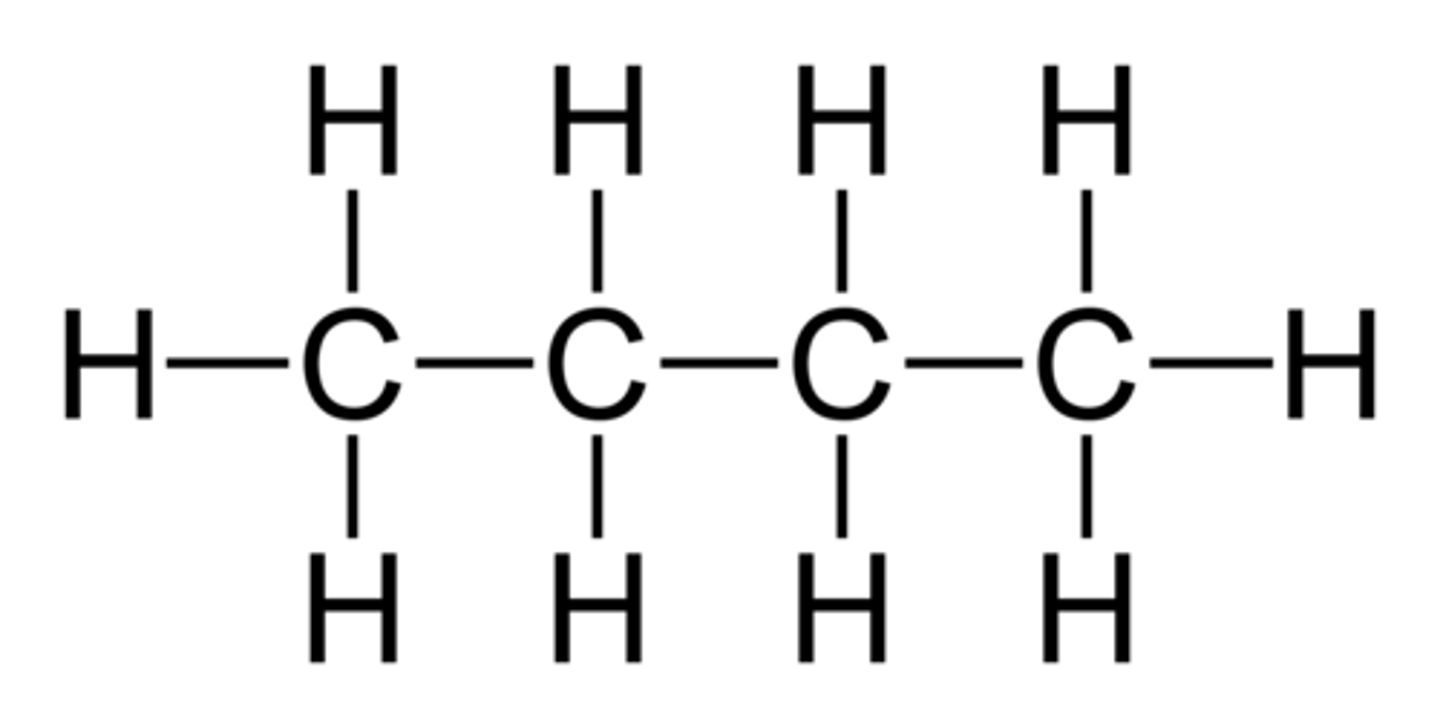
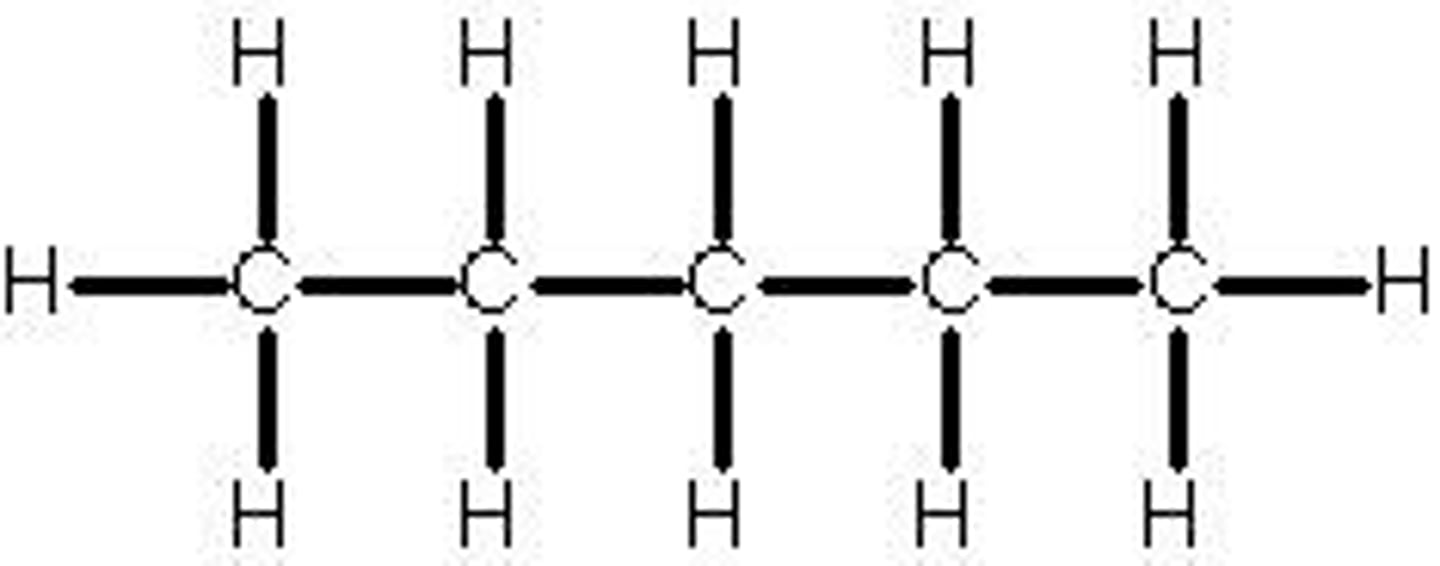
Pentane
C5H12
Ethene
C2H4
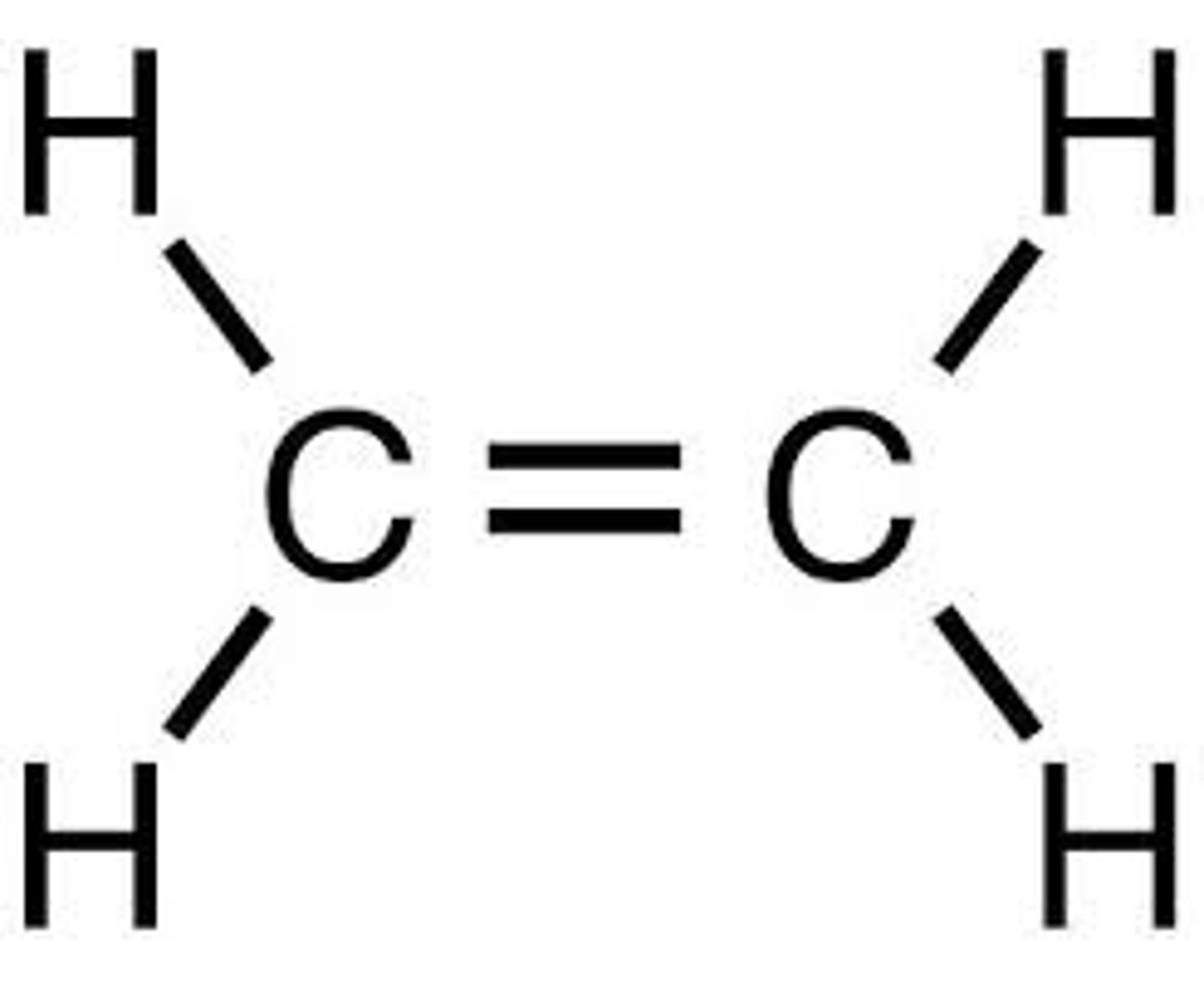
Propene
C3H6
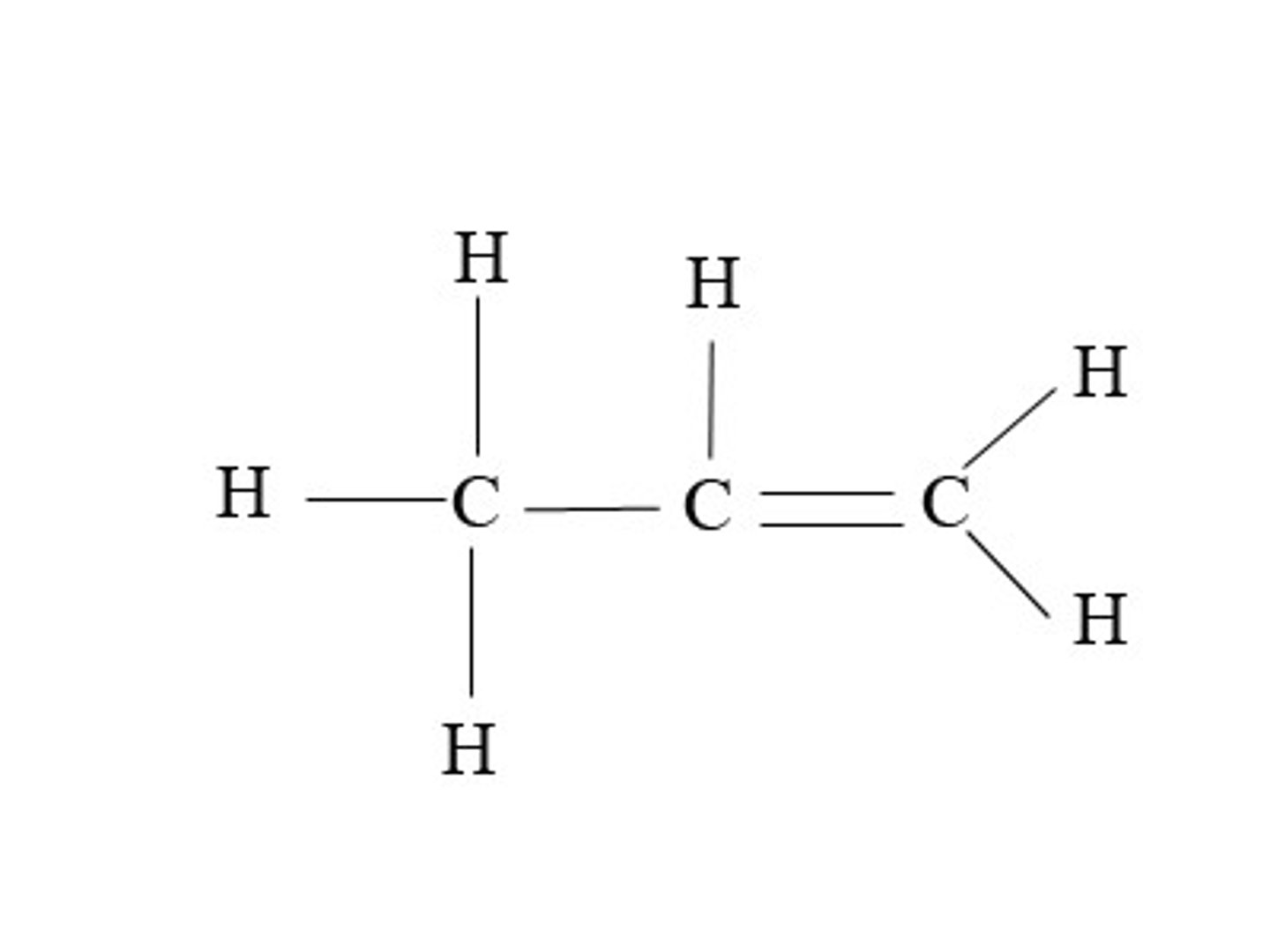
Butene
C4H8
Ethyne
C2H2
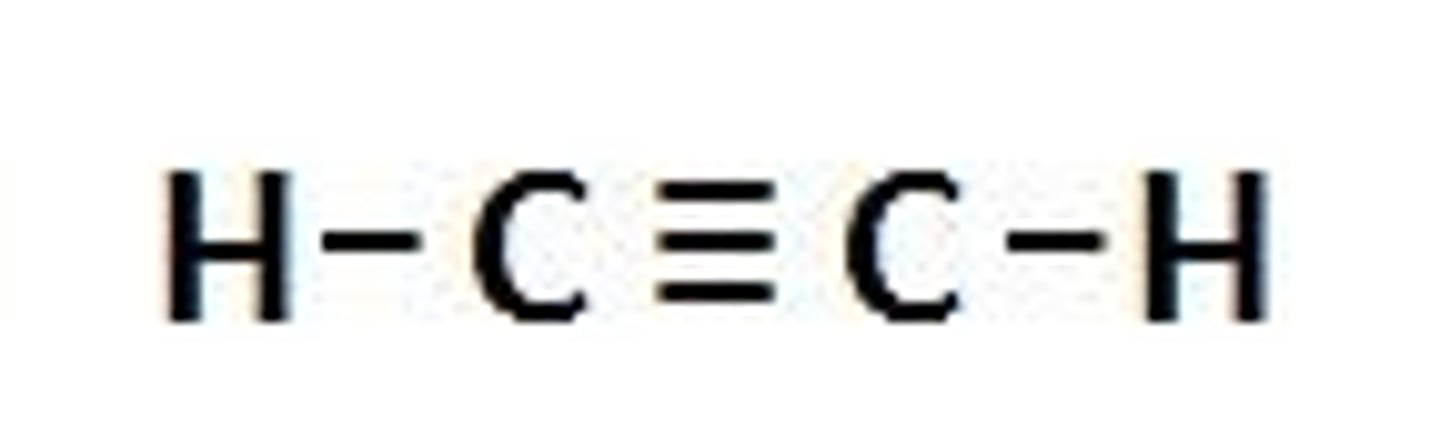
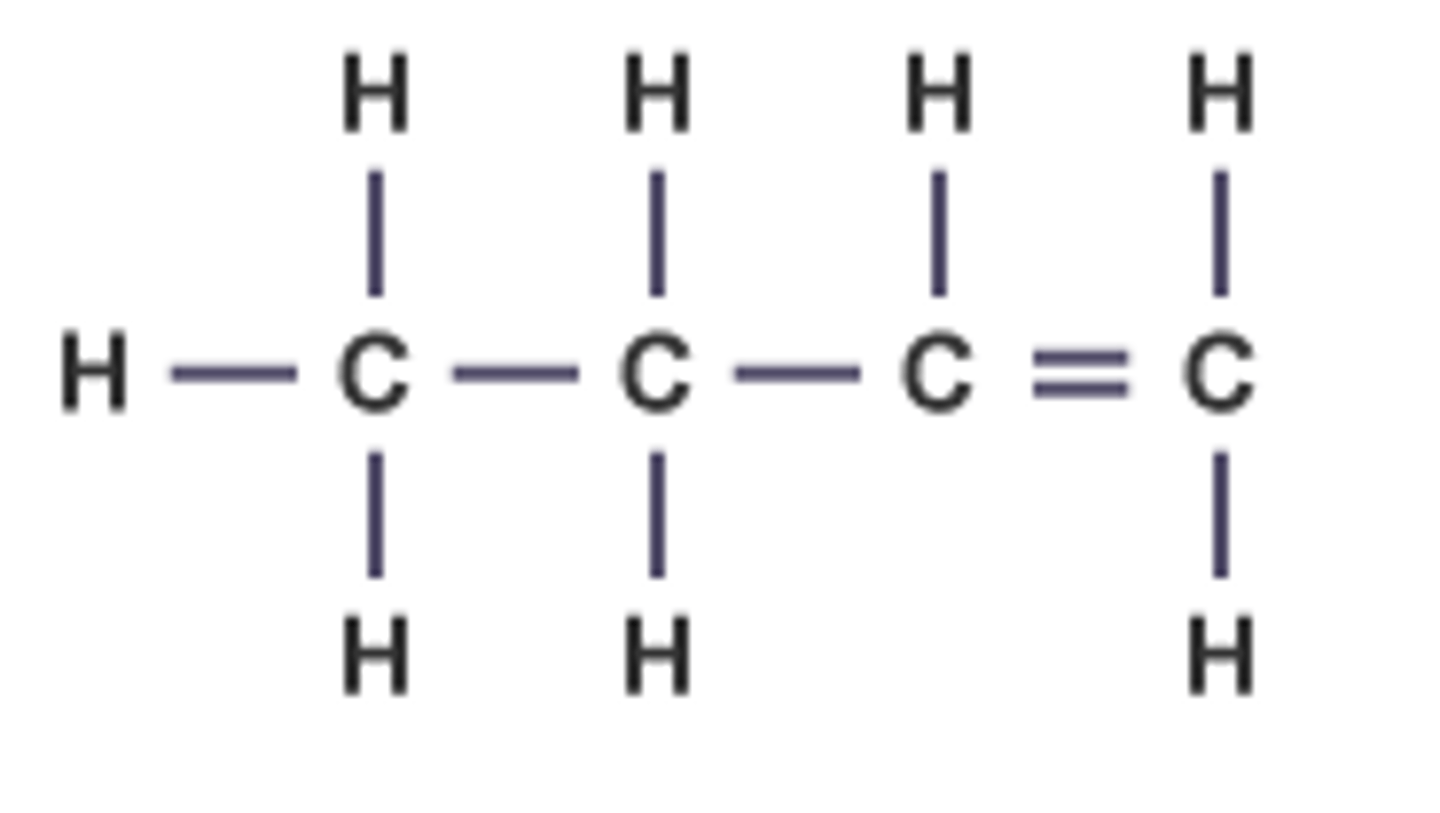
Propyne
C3H4
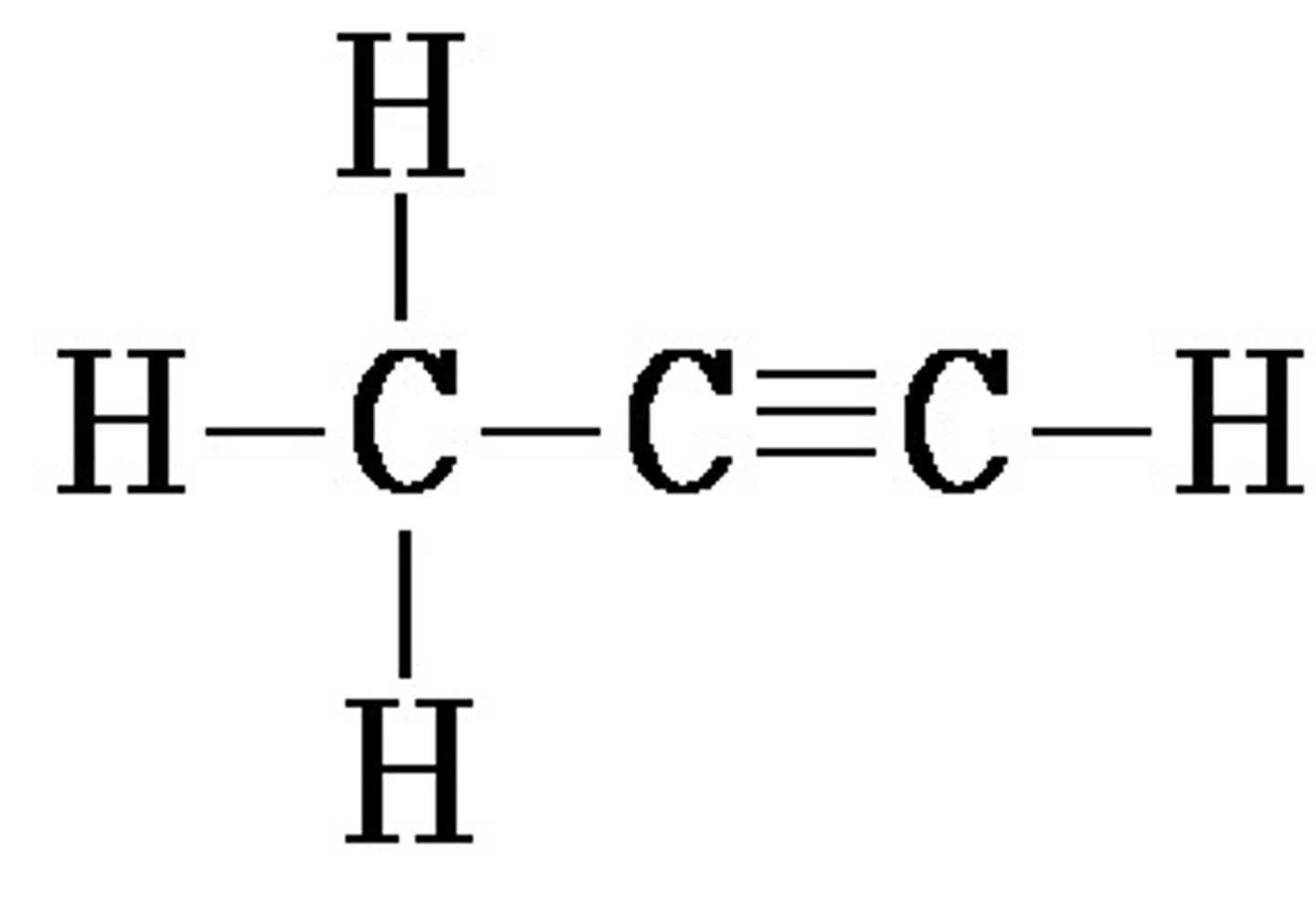
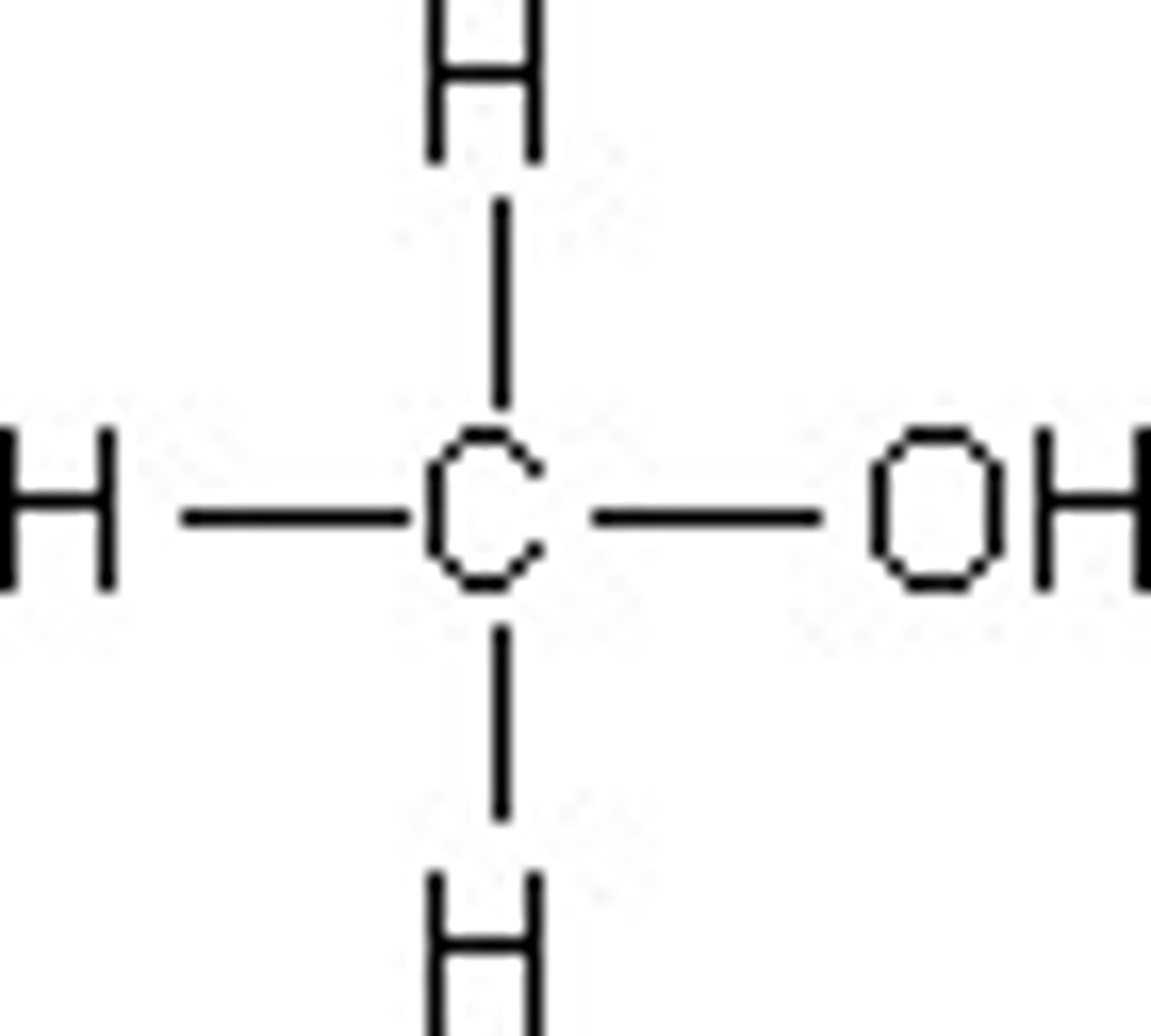
Methanol
CH3OH
Ethanol
C2H5OH
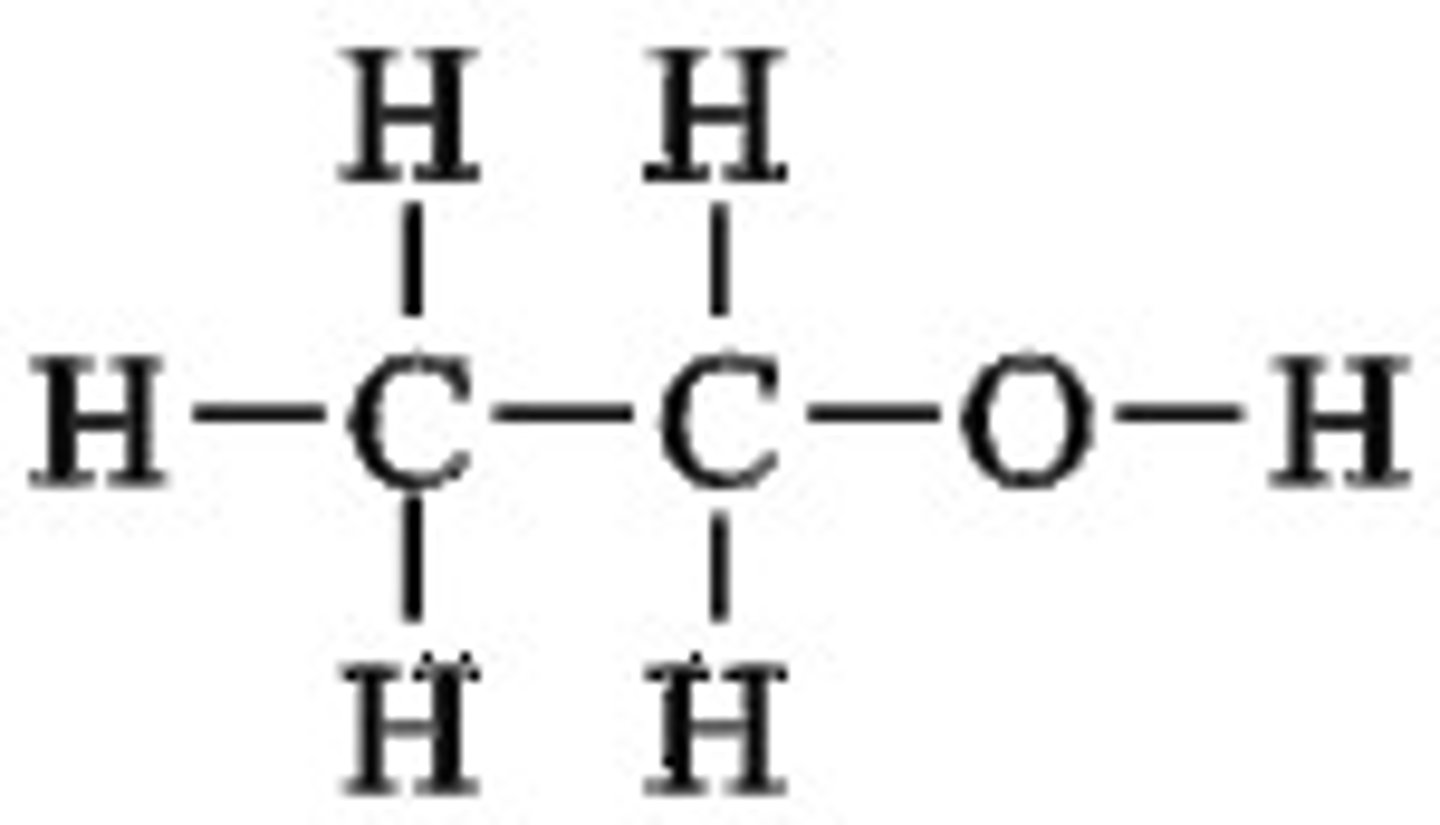
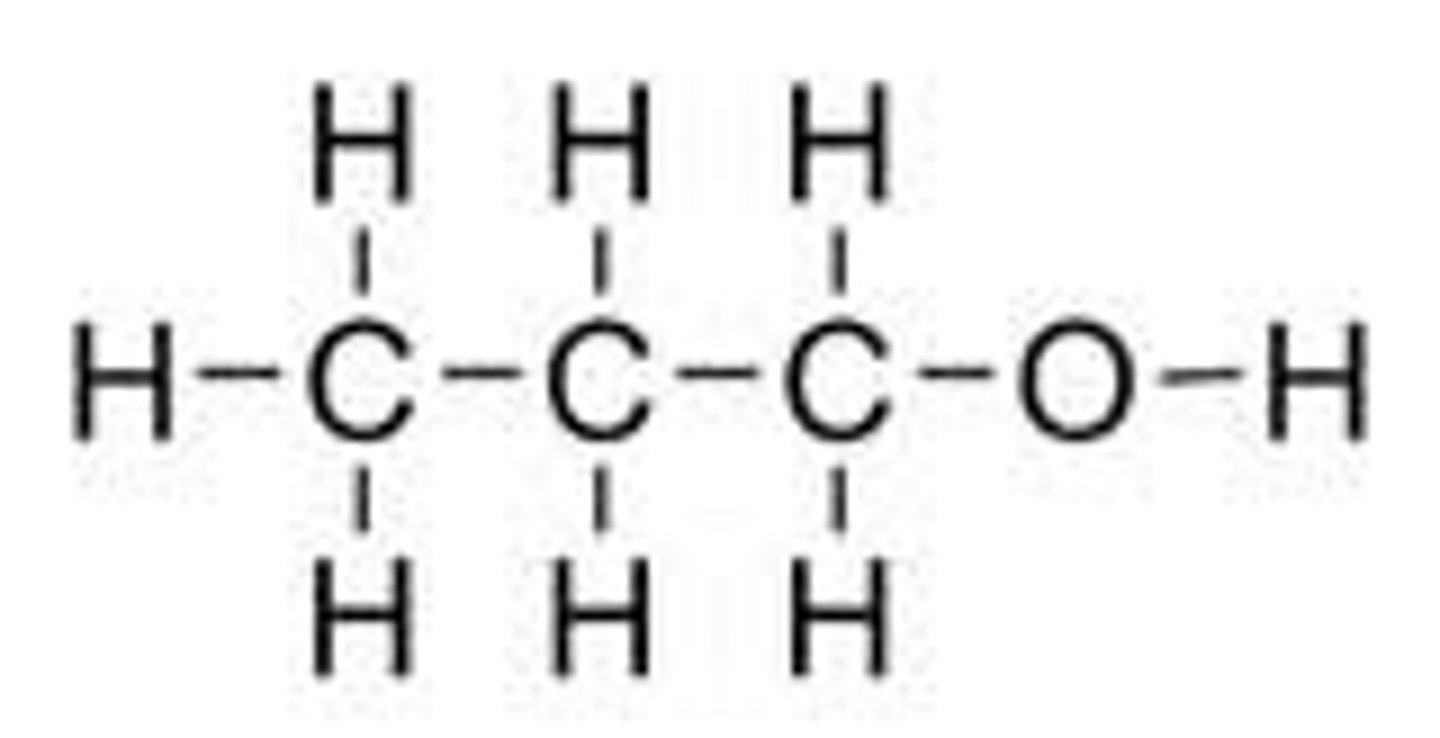
Propanol
C3H7OH
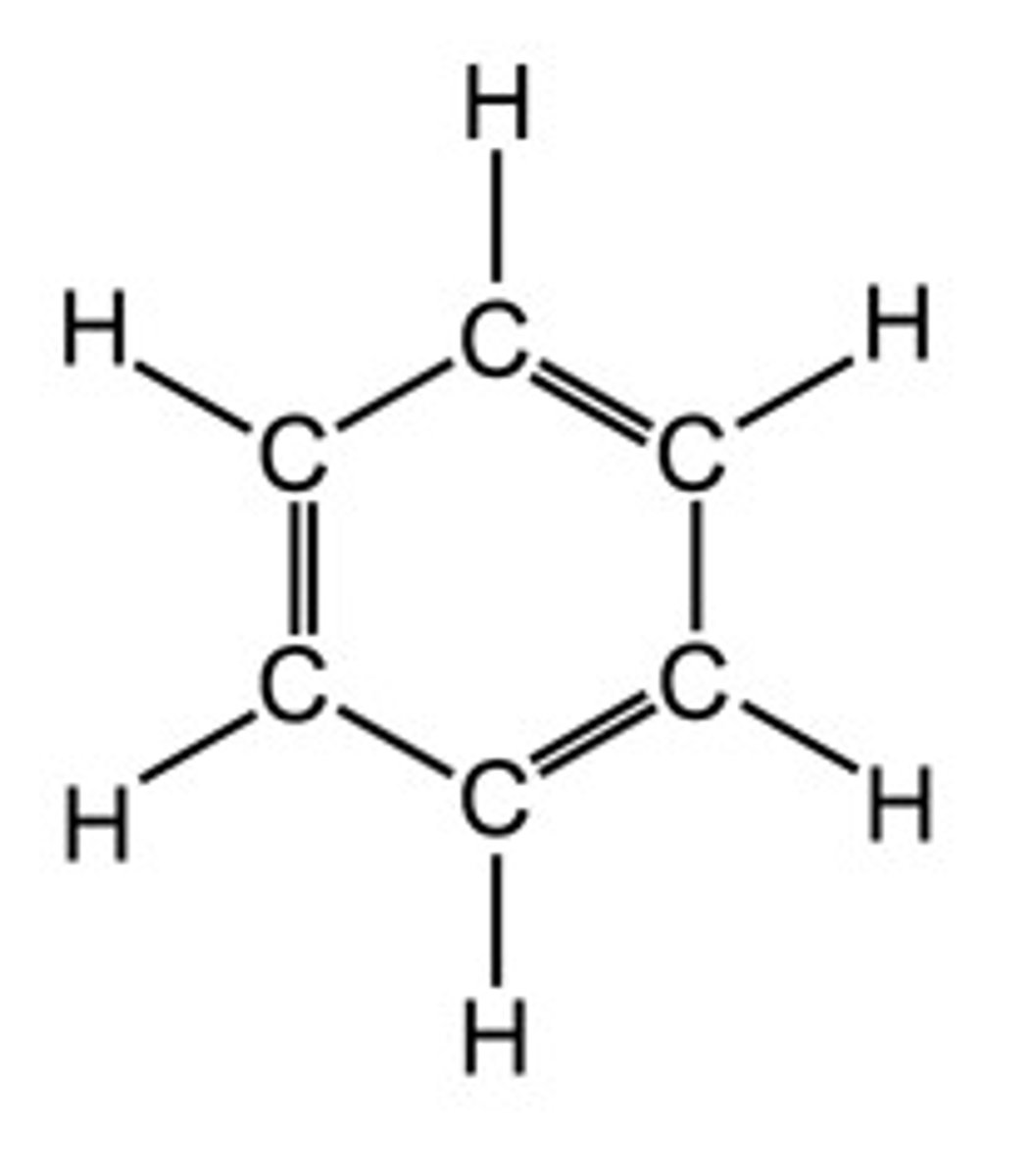
Benzene
C6H6
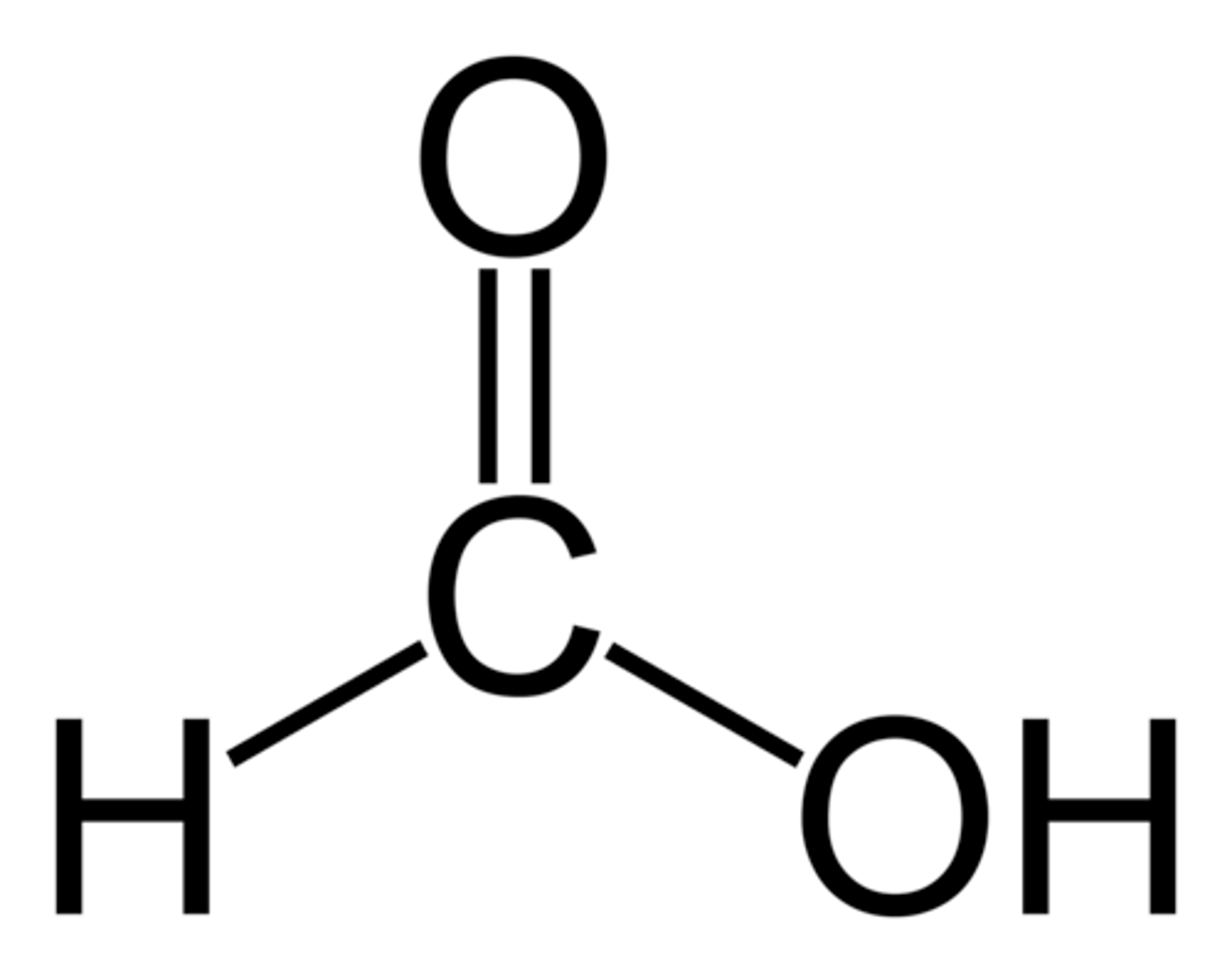
formic acid (methanoic acid)
HCOOH
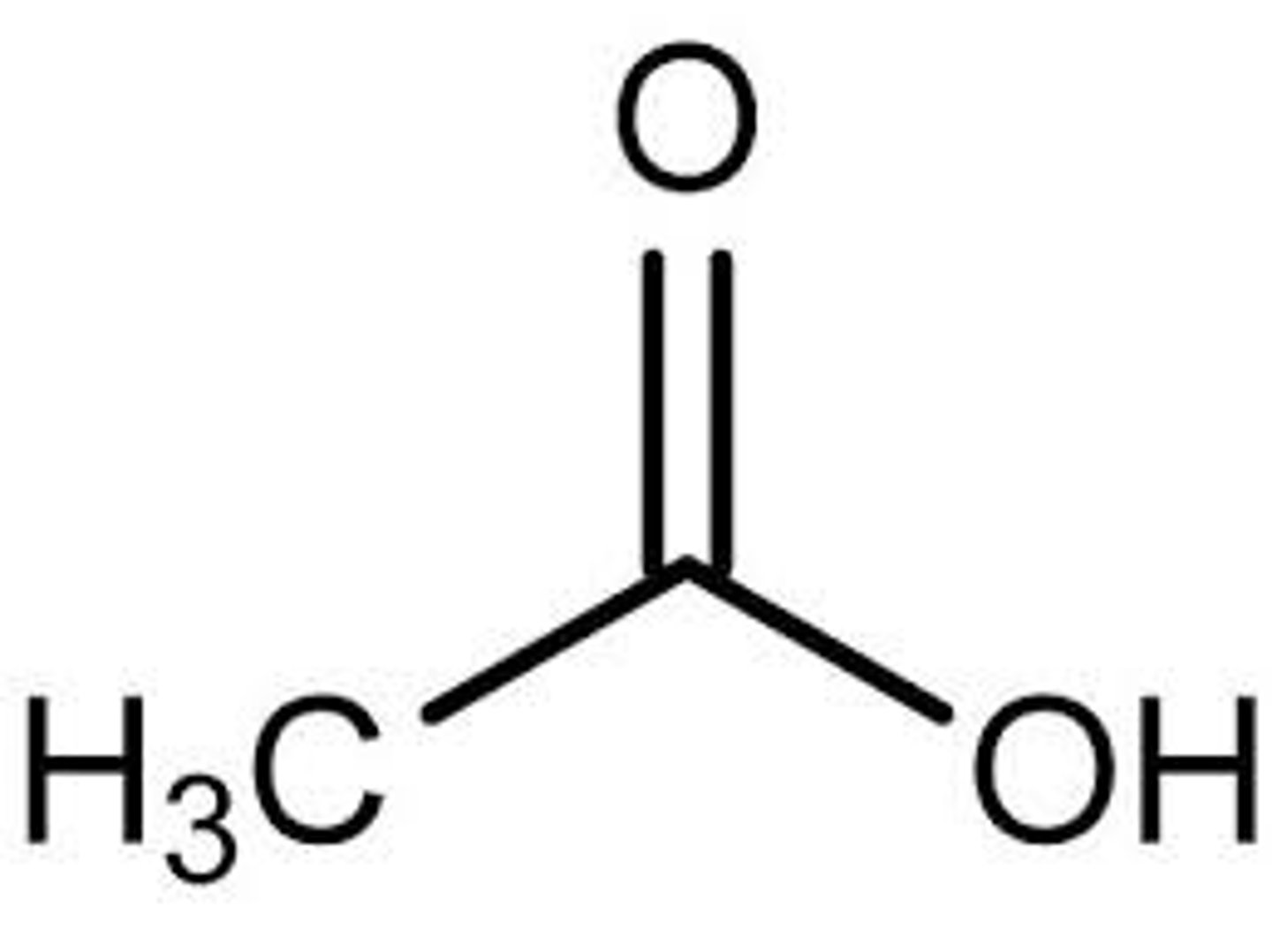
Acetic Acid (Vinegar)
CH3COOH
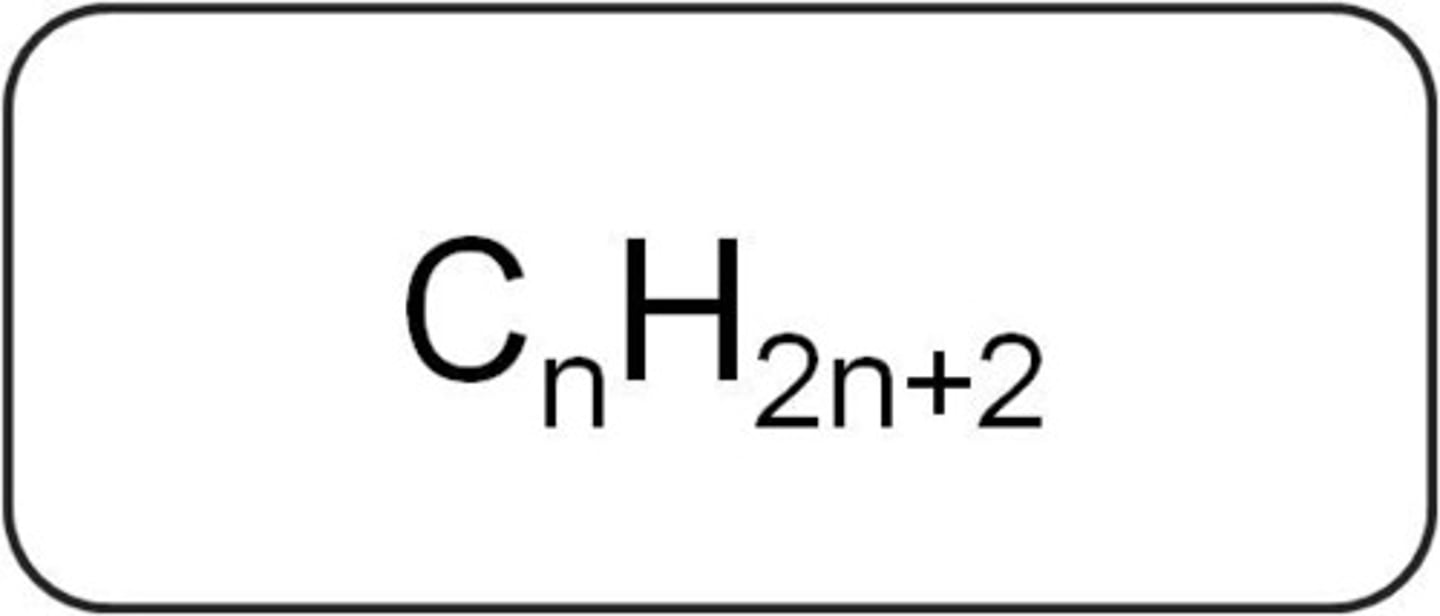
Formula for an alkane
CnH2n+2
Formula for an alkene
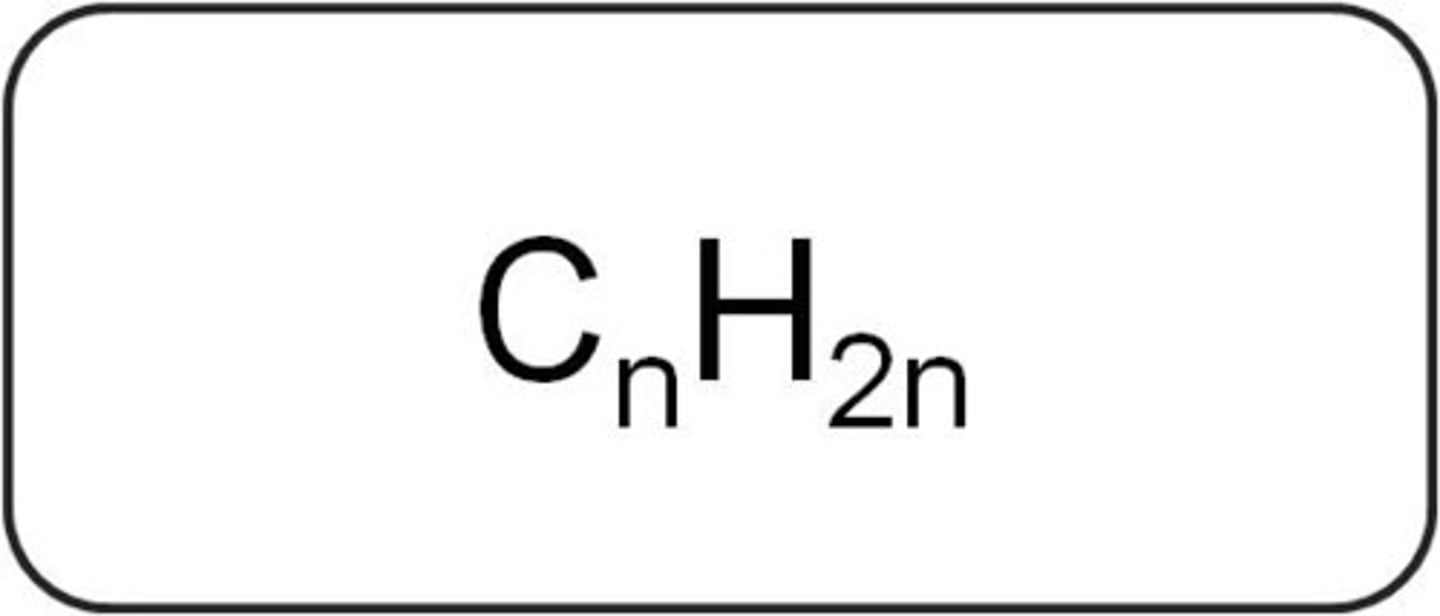
CnH2n
Formula for an alkyne
CnH2n-2