UNC BIOL253 Evaluation 2 Study Guide
1/86
Earn XP
Description and Tags
Content on cell membranes, diffusion, osmosis, membrane potentials, and neurophysiology
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
87 Terms
Functions of plasma membranes
Regulate the passage of substances into and out of cells and between cell organelles and cytosol
Detect chemical messengers arriving at the cell surface
Link adjacent cells together by membrane junctions
Anchor cells to the extracellular matrix
Structure of plasma membrane
made of phospholipid bilayer with embedded proteins (amphipathic integral or transmembrane proteins) and peripheral proteins (along surfaces of the membrane)
Glycerol
Fatty acids
Phosphate groups
Phospholipids
Proteins
Cholesterol
Fluid-mosaic model
concept of proteins freely moving about in the lipid bilayer of plasma membrane
Amphipathic
a molecule that has both a hydrophilic (water-loving/polar/charged) region, and a hydrophobic (water-repelling/nonpolar) region within the same molecule
spontaneously arrange themselves in water so that hydrophilic parts face water, and hydrophobic parts avoid water
Ex. phospholipids
Transmembrane proteins (integrins)
Proteins that span the entire plasma membrane → part of the protein is inside the cell, while part is embedded inside the lipid bilayer, and another part extends outside the cell
Extracellular matrix (ECM)
A network of proteins and carbohydrates outside cells that provides structural support and biochemical signals to tissues
The role of integrins binding to the extracellular matrix
Physical attachment: They form a mechanical link between the ECM and the cell interior (this is how cells stay anchored in tissues)
Signal transduction (outside ←→ inside): integrins change shape and trigger intracellular signaling pathways for cells to receive information about mechanical stress, ECM composition, and tissues
Cell junctions
How are cells packed together into tissue/organ types and how do they interact with neighboring cells/tissue?
Cells pack together using junctions, ECM, and membrane proteins
Interactions occur through:
Direct contact
Chemical signals
Mechanical forces
The structure of the tissue reflects its function
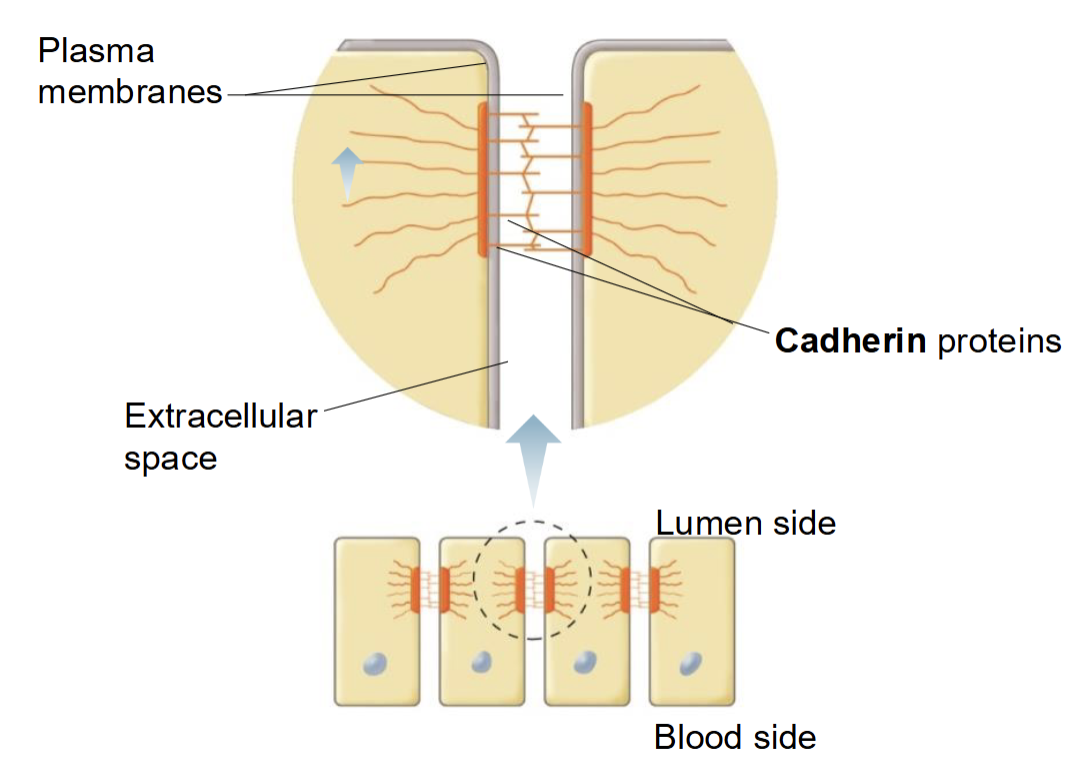
Desmosomes (anchoring junction)
Dense plaques (accumulated proteins), Cadherin proteins join plasma membranes of adjacent cells together, found in tissue subject to considerable stretching
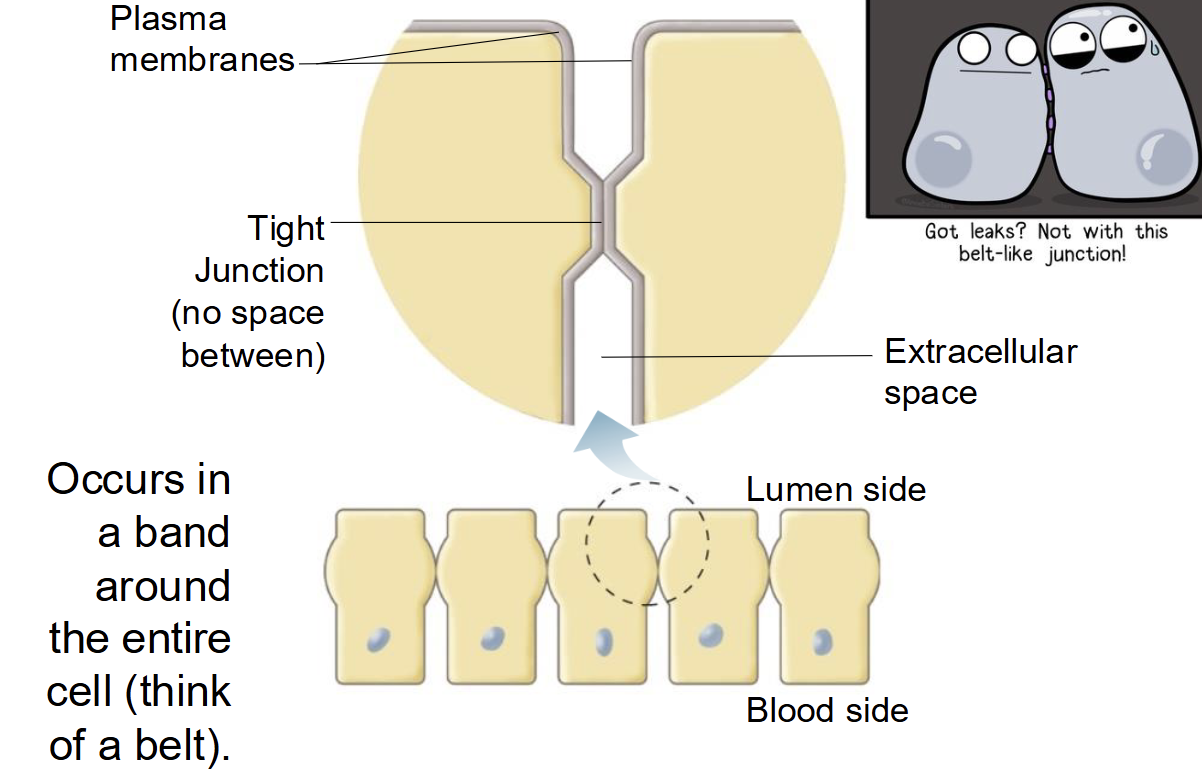
Tight junction (occluding junction)
no space in between adjacent cells, occurs in a band around the entire cell (think like a belt), found in most epithelial cells, prevents paracellular movement of substances
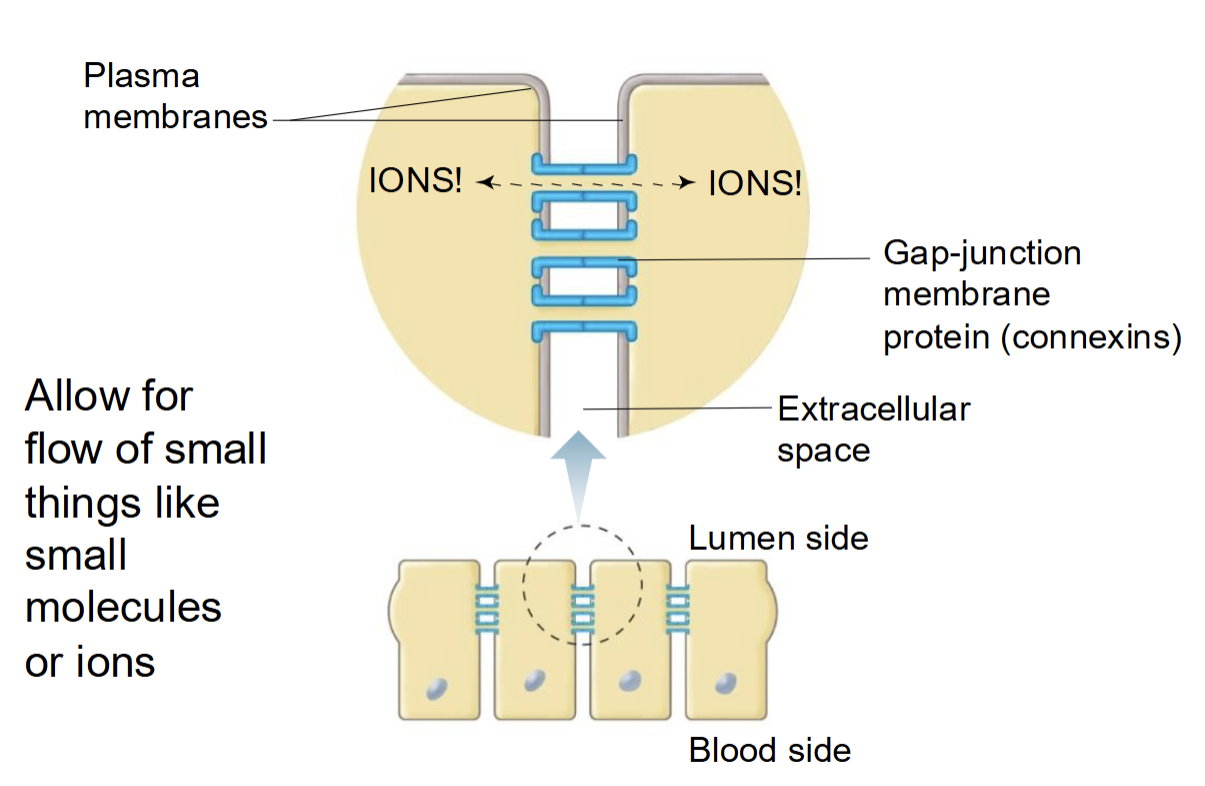
Gap junction (communicating junction)
membrane proteins called connexins join cells together, allows for flow of small things like ions or small molecules, helps with electrical signal flow
Occluding junctions
seal cells together in an epithelium (prevents small molecules from leaking from one side to the other)
Anchoring junctions
mechanically attach cells (and their cytoskeleton) to their neighbors or the extracellular matrix
Communicating junctions
mediate passage of chemical or electrical signals from one interacting cell to its neighbor
How fast does plasma membrane turnover happen?
In minutes to hours
Factors that influence the rate of diffusion
Concentration gradient
Distance (path length) → thicker membranes or tissues slow diffusion down
Surface area → greater surface area = faster diffusion
Molecule size (molecular weight) → smaller molecules diffuse faster than larger ones
Temperature → high temperature = more kinetic energy = faster diffusion
Medium → diffusion is fastest in gases, slower in liquids, and slowest in solids
Membrane permeability
Electrical gradient (for ions)
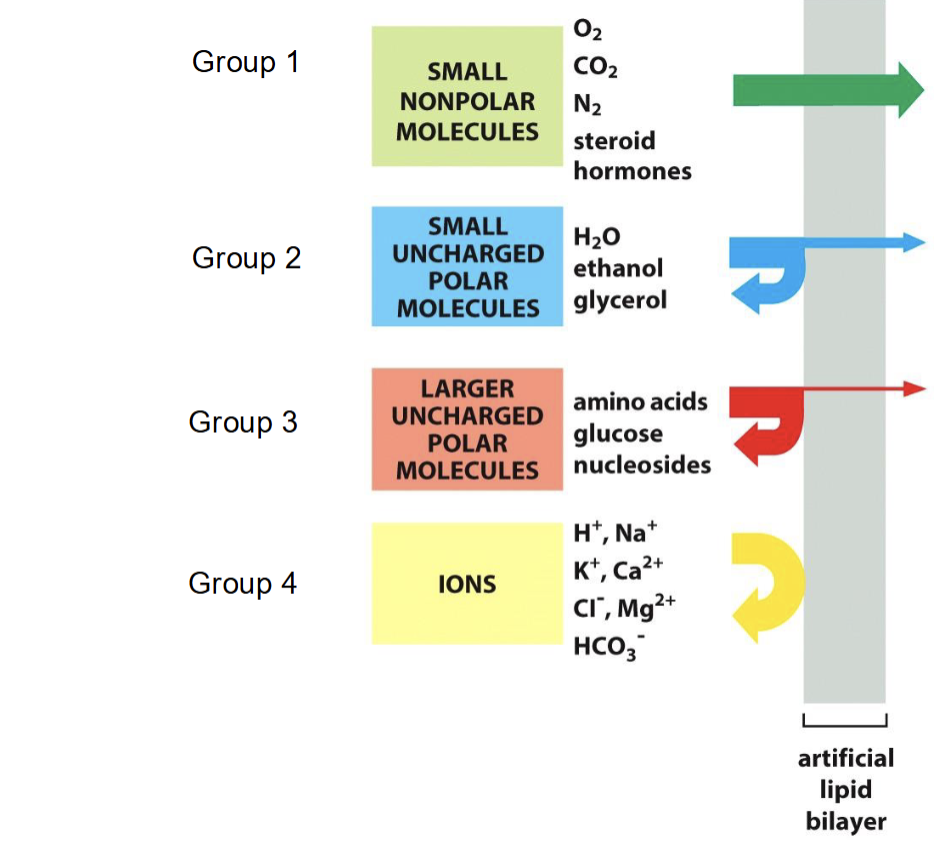
What type of ions freely pass through the phospholipid bilayer?
Freely: small, nonpolar molecules (O2, CO2, N2, steroid hormones)
Group 2: small, uncharged polar molecules (water, ethanol, glycerol)
Group 3: larger, uncharged polar molecules (amino acids, glucose, nucleosides)
Group 4: Ions (H+, Na+, K+, Ca2+, Cl-, Mg2+, HCO3-)
Osmosis
net diffusion of water
Osmolarity
total solute concentration of a solution
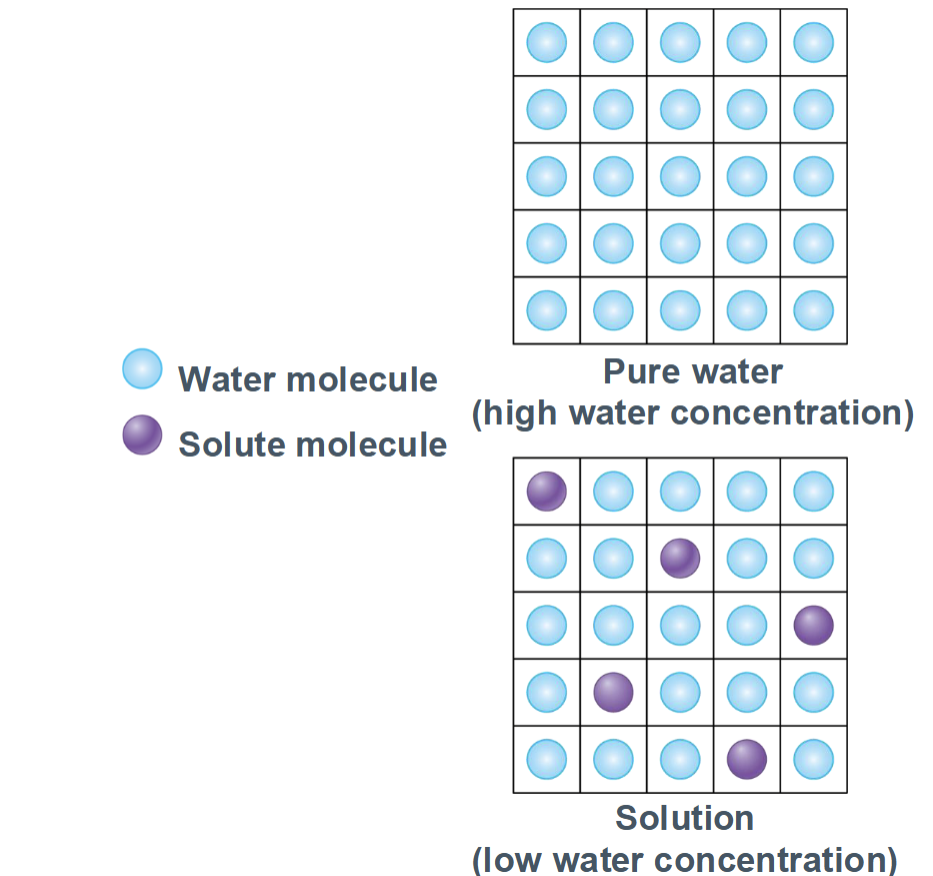
Membrane permeability vs. osmotic pressure
Only solutes that cannot cross the membrane create lasting osmotic pressure
depends on the number of non-penetrating solutes and the membrane permeability to those solutes
Membrane permeability vs. volume changes
Occurs when a membrane is permeable to water but not solutes
solutes are trapped on one side
Water moves towards higher solute concentration
Causes cell to shrink (hypertonic) or swell (hypotonic)
Occurs when a membrane is permeable to some solutes
Permeable solutes diffuse across the membrane
Osmotic pressure decreases as solute concentrations equalize
Penetrating solutes do not sustain osmotic pressure
Tonicity
he ability of a solution to change the volume of a cell by causing water to move across a semipermeable membrane
depends only on non-penetrating (impermeable) solutes
describes the effect on cell volume, not just solute concentration
Types include isotonic, hypertonic, and hypotonic
Isotonic
no change in the cell volume (equal concentrations outside and inside the cell)
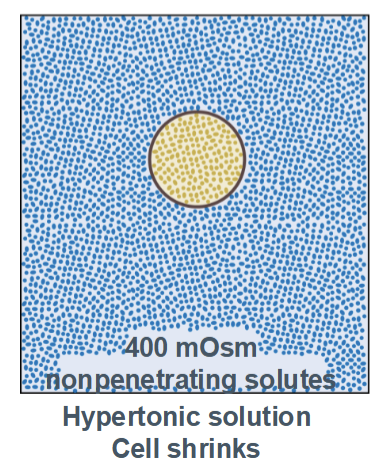
Hypertonic
cell shrinks (higher concentration in the environment than inside the cell)
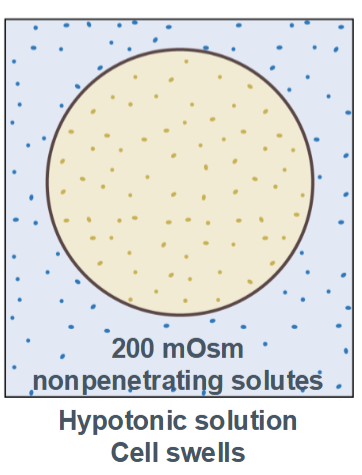
Hypotonic
cell swells with water (higher concentration inside the cell than in the environment)
Examples of osmosis in healthcare
IVs, kidney function, dialysis, digestion/nutrition, eye care, diuretics
Membrane potential
The electrical voltage difference across a cell’s plasma membrane, caused by an unequal distribution of charged ions between the inside and outside of the cell
Measured in millivolts (mV)
The inside of the cell is usually negative relative to the outside
Typical resting membrane potential: neurons ~ -70mV, muscle cells ~ -90mV
Membrane permeability to specific ions
At rest, membranes are most permeable to K⁺
K⁺ leaks out through leak channels → inside becomes negative
Opening or closing ion channels changes permeability and membrane potential
Examples:
↑ Na⁺ permeability → depolarization
↑ K⁺ permeability → hyperpolarization
↑ Cl⁻ permeability → stabilizes or hyperpolarizes membrane
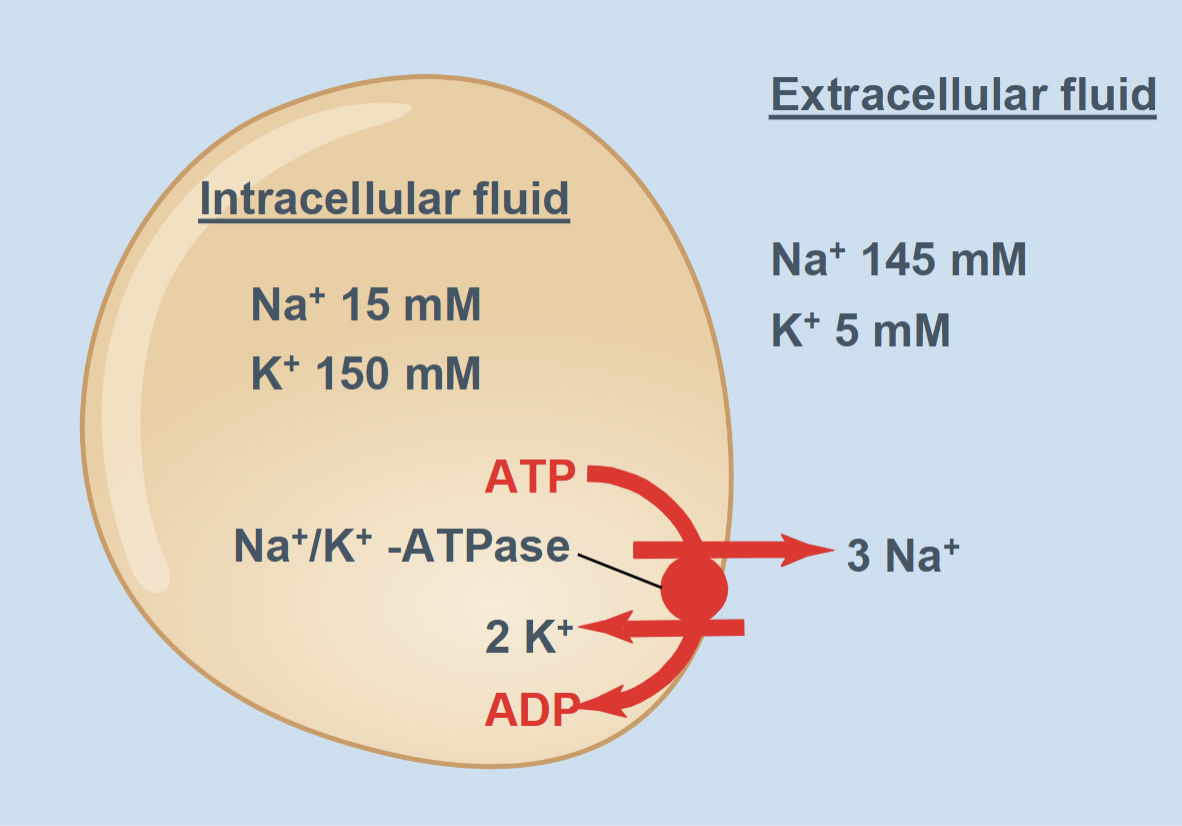
Electrogenic pump (Na+/K+-ATPase)
Moves 3 Na+ out and 2 K+ in, creates and maintains ion gradients, and slightly contributes to negativity inside the cell
What are the two driving forces of the cellular electrochemical gradient?
Concentration gradient: difference in ion concentration across the membrane, ions move from high to low concentration
Electrical gradient: difference in electrical charge across the membrane, ions move toward opposite charges
Why might an H+-ATPase (proton pump) be useful in cells?
actively transports protons (H⁺) across a membrane using energy from ATP, creating proton gradients that the cell can use for multiple vital functions
Generating an electrochemical gradient: Pumps H⁺ out of the cytoplasm
Driving secondary transport: The proton gradient can be used to drive the transport of other molecules against their concentration gradient
H⁺ moving back down its gradient can co-transport nutrients (e.g., glucose, amino acids)
Maintaining intracellular pH: Pumps H⁺ out of the cytoplasm to prevent acidification and keeps enzyme function and metabolism optimal
Why might an H+/K+-ATPase be found in acid secreting cells of the stomach?
it actively pumps protons (H⁺) into the stomach lumen in exchange for potassium (K⁺), creating the highly acidic environment needed for digestion
Mediated transport
the movement of molecules across a cell membrane with the help of specific proteins, because the molecules are too large, charged, or polar to diffuse freely through the lipid bilayer
Facilitated diffusion
Direction: Down the concentration gradient (no energy required)
Proteins involved: Carrier proteins or channels
Examples and tissue specialization:
Glucose transporters (GLUTs) in muscle and liver → allow glucose to enter cells when blood glucose is high
Selective and faster than simple diffusion, but cannot move against a gradient
Primary active transport
Direction: Against the concentration gradient (requires energy from ATP)
Proteins involved: Pumps (ATPases)
Examples and tissue specialization:
Na⁺/K⁺-ATPase in all cells → maintains resting membrane potential and osmotic balance
H⁺/K⁺-ATPase in stomach parietal cells → secretes acid for digestion
Generates ion gradients used for signaling, volume control, and secondary transport
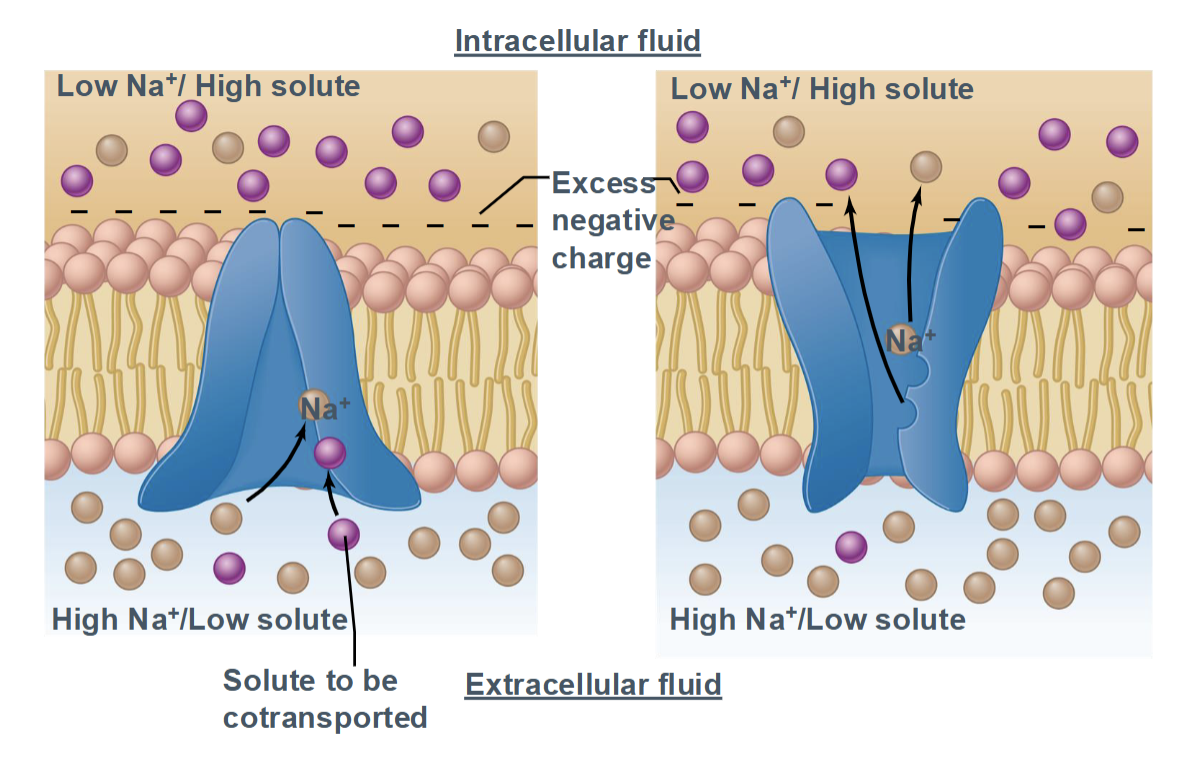
Secondary active transport
Direction: Uses the gradient of one molecule to drive movement of another
Proteins involved: Symporters or antiporters
Examples and tissue specialization:
SGLT (sodium–glucose cotransporter) in kidney and intestine → uses Na⁺ gradient to absorb glucose
Na⁺/Ca²⁺ exchanger in cardiac muscle → removes calcium for muscle relaxation
Relies on gradients established by primary active transport; efficient for nutrient absorption and ion homeostasis
Fanconi-Bickel Syndrome
No underlying enzymatic defect in carbohydrate metabolism had been identified, metabolism of both glucose and galactose is impaired, effects GLUT2 deficiency (SLC2A2 mutation), glycogen storage disease
Epithelial transport
the movement of substances across an epithelial cell layer, from one side of the tissue to the other (for example, from the intestinal lumen into the blood)
Transcellular pathway
Paracellular pathway
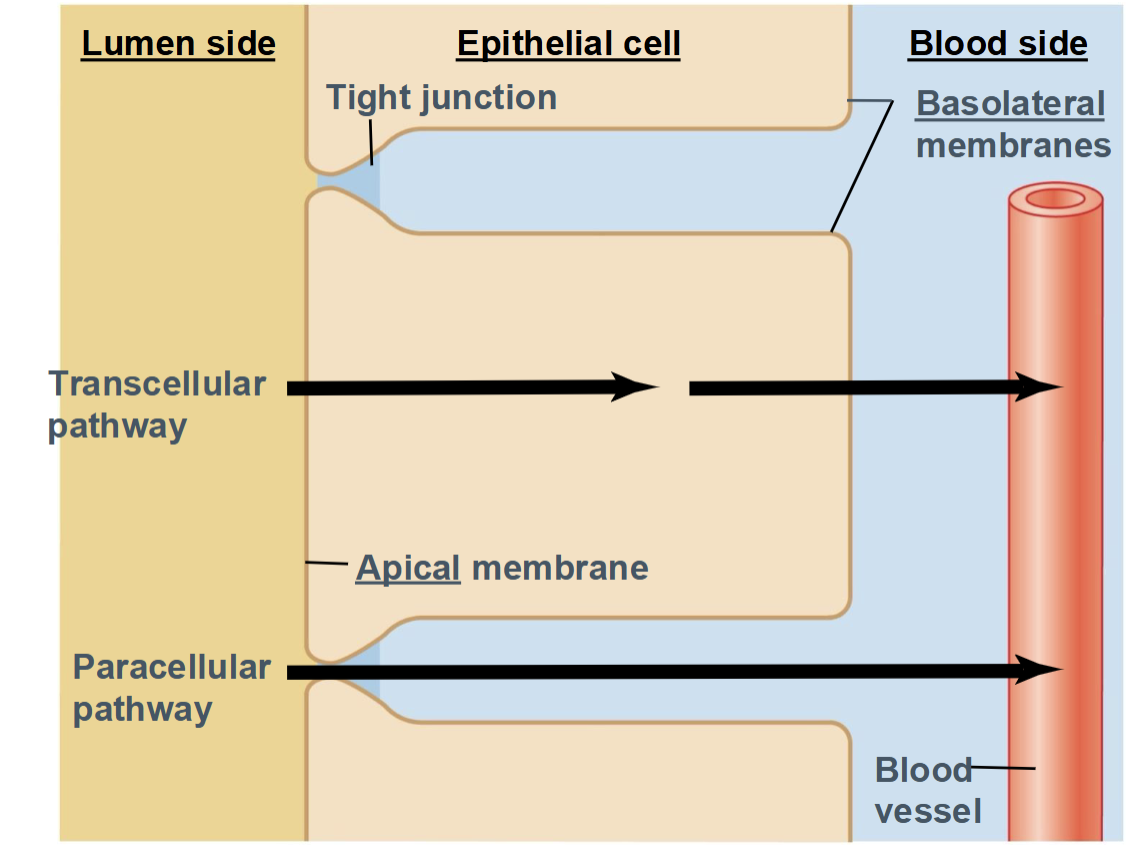
Transcellular pathway
Substance moves through the epithelial cells
Uses channels, carriers, and pumps
Highly selective and regulated
Paracellular pathway
Substance moves between cells
Passes through tight junctions
Usually limited to small ions or water
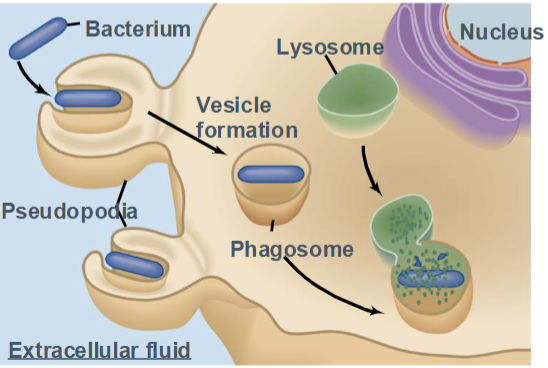
Phagocytosis
a form of endocytosis in which specialized cells engulf large particles, such as bacteria, dead cells, or debris.
Known as “cell eating”
Performed mainly by immune cells (macrophages, neutrophils)
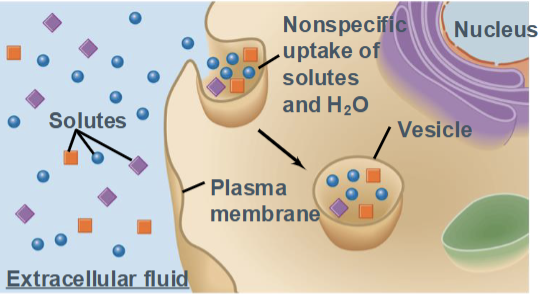
Pinocytosis
a form of endocytosis in which a cell nonspecifically engulfs extracellular fluid and dissolved solutes into small vesicles.
Often called “cell drinking”
Occurs continuously in most cells
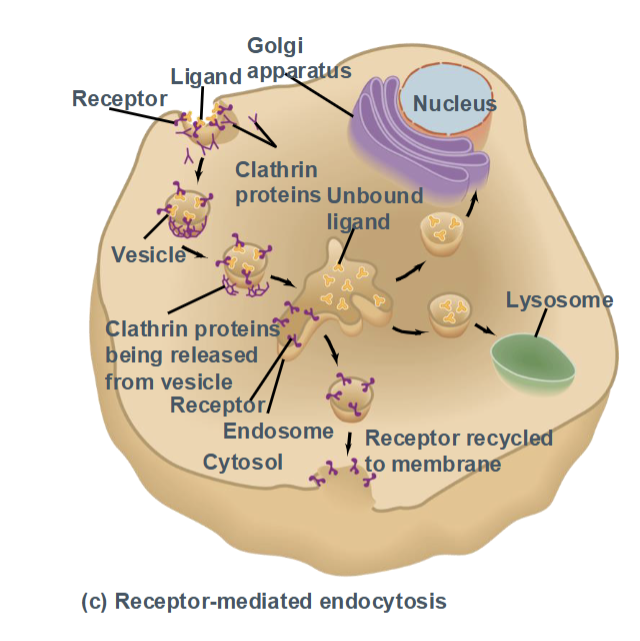
Receptor-mediated endocytosis
a highly specific form of endocytosis in which ligands bind to cell-surface receptors, triggering vesicle formation.
Uses clathrin-coated pits
Allows efficient uptake of specific molecules (e.g., LDL cholesterol, hormone
Exocytosis
the process by which a cell releases substances to the outside by fusion of an intracellular vesicle with the plasma membrane.
Used to secrete hormones, neurotransmitters, enzymes, and membrane proteins
Can be constitutive (continuous) or regulated (stored then released)
Endocytosis
the process by which a cell brings substances into the cell by forming a vesicle from the plasma membrane.
Requires energy
Includes:
Pinocytosis
Phagocytosis
Receptor-mediated endocytosis
Trogocytosis
a process in which a cell extracts and internalizes small pieces of another cell’s plasma membrane during direct cell–cell contact.
Common in immune cells (T cells, NK cells)
Allows rapid cell-to-cell communication and signaling modulation
Apical membrane
faces fluid cavities, body surfaces
Basolateral membrane
lateral surfaces face other epithelial cells; basal cells face connective tissues
Volts (V)
difference in charge across a membrane
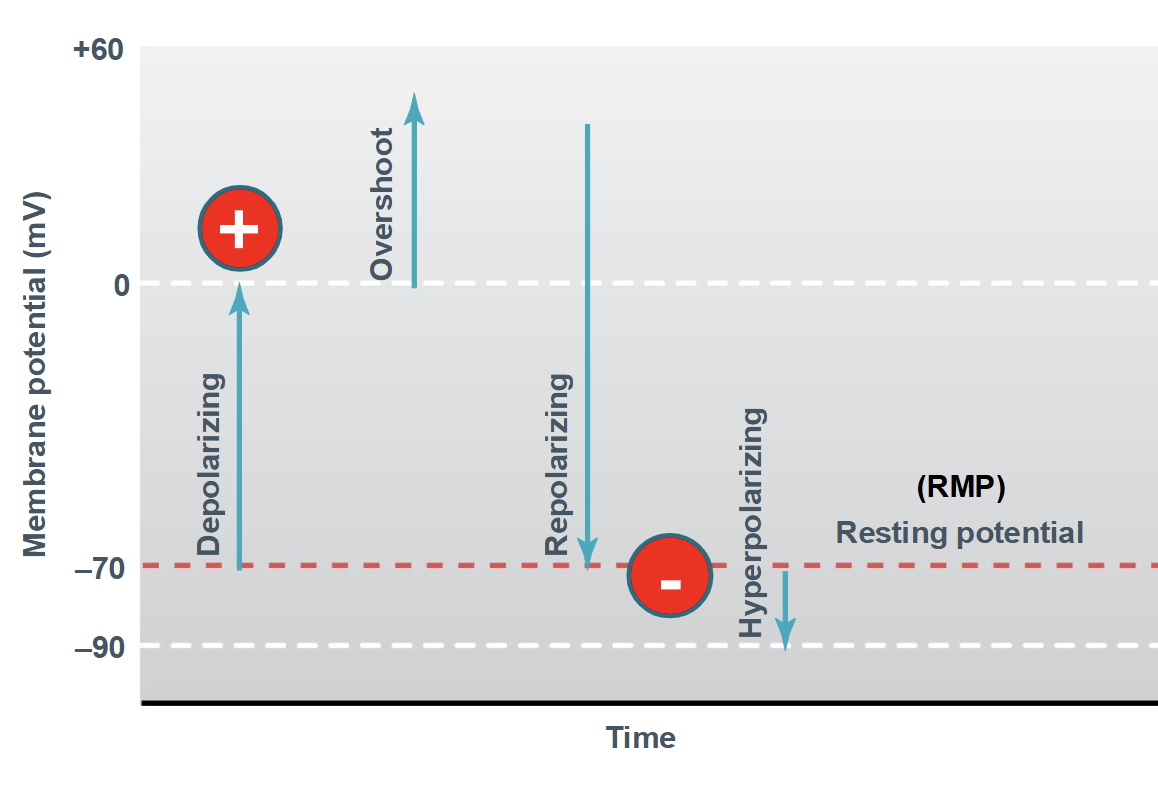
Resting membrane potential
the stable electrical voltage across the plasma membrane of a non-signaling cell, with the inside of the cell being negative relative to the outside.
Typical values:
Neurons: ~ −70 mV
Muscle cells: ~ −90 mV
How resting membrane potential is maintained
1. Unequal ion distribution
Na⁺: high outside, low inside
K⁺: high inside, low outside
Cl⁻: high outside
Negatively charged proteins trapped inside the cell. These gradients create a tendency for ions to move.
2. Selective membrane permeability
At rest, the membrane is most permeable to K⁺
K⁺ leaks out through K⁺ leak channels
Loss of positive charge leaves the inside negative
Electrical forces balance diffusion
As K⁺ leaves, the interior becomes negative
Electrical attraction pulls K⁺ back in
Equilibrium between chemical and electrical forces stabilizes the voltage
Na⁺/K⁺-ATPase (ion pump)
Pumps 3 Na⁺ out and 2 K⁺ in using ATP
Maintains ion gradients
Slightly increases the negative charge inside the cell
Membrane potential (Vₘ)
the electrical voltage difference across the plasma membrane, resulting from unequal distribution of ions and selective membrane permeability.
Expressed in millivolts (mV)
Inside of the cell is typically negative relative to the outside
Equilibrium potential
the membrane voltage at which there is no net movement of a specific ion across the membrane, because the electrical force exactly balances the chemical (concentration) gradient for that ion.
Each ion has its own equilibrium potential (e.g., Eₖ, Eₙₐ)
Calculated using the Nernst equation
Electrogenic pump
a membrane transport protein that moves unequal numbers of charges across the membrane, directly contributing to the membrane potential.
Requires ATP
Example: Na⁺/K⁺-ATPase (3 Na⁺ out, 2 K⁺ in)
How does the Na/K pump contribute to the membrane potential?
It established the concentration gradient and creates a small negative potential
Greater net movement of K+ out makes membrane more negative inside the cell
Steady negative resting membrane potential, ion flux through channels balances each other
Overshoot
peak of positive potential (depolarization above 0 mV)
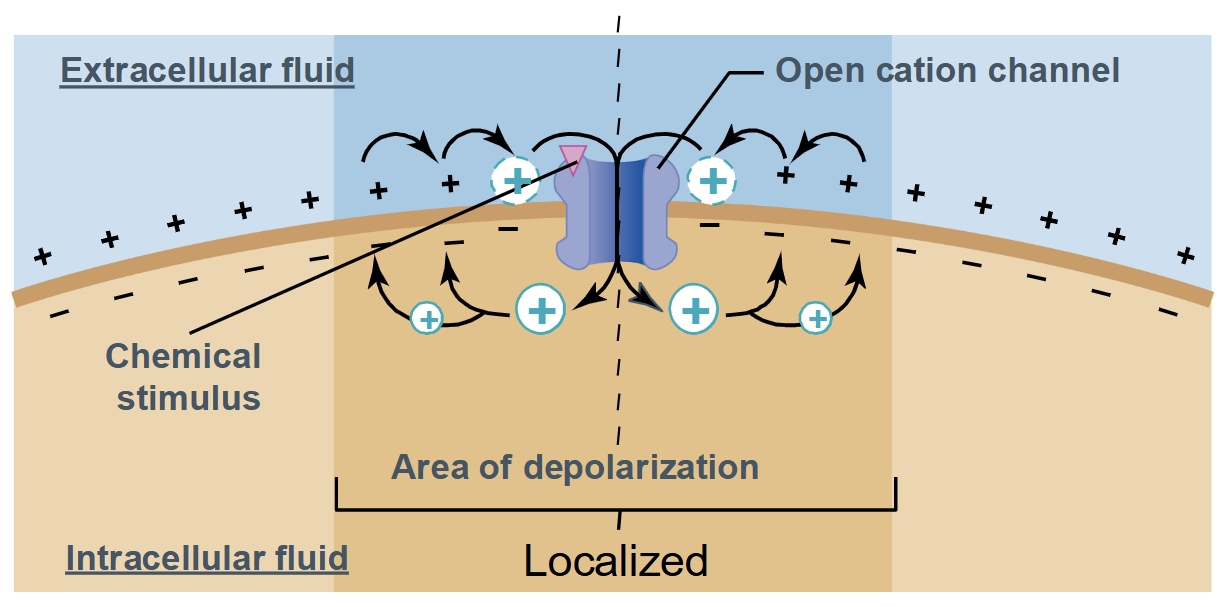
Graded potentials
Occurs in a small region of the plasma membrane (localized)
Magnitude can vary
Decremental signals → become weaker as they get further from the origin
No threshold and no refractory period
Action Potentials
Large alterations in membrane potential
Rapid and repeating
Long-distance cell communication
Voltage-gated ion channels
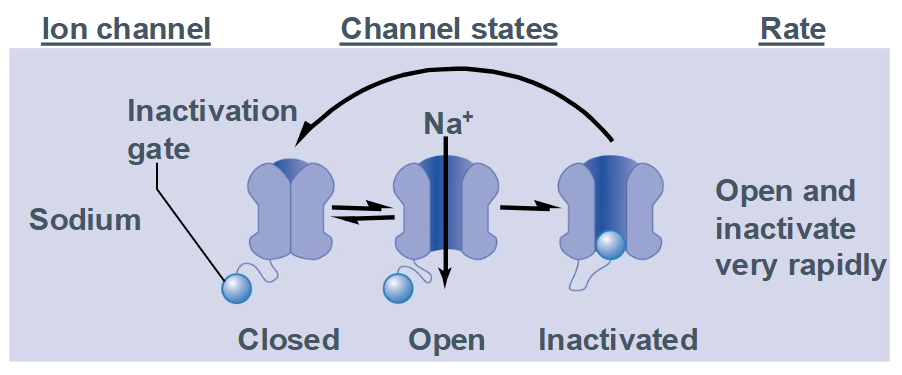
Characteristics of Na+ Channels
Channel has 3 states:
closed (resting)
open
inactivated (open but inactivation gate is blocking the channel)
Opens and inactivates very rapidly
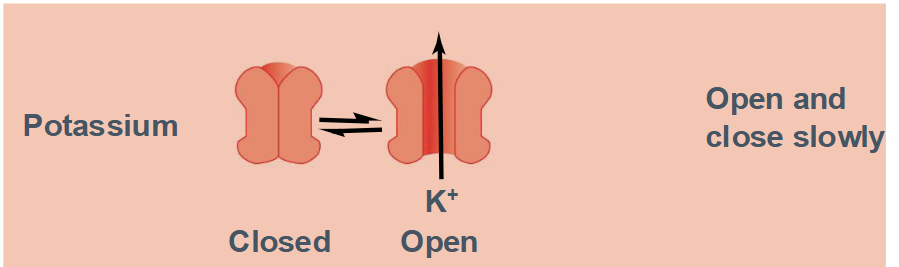
Characteristics of K+ Channels
Channel has 2 states:
Open
Closed
Opens and closes slowly
Steps of an Action Potential
Steady resting membrane potential is near Ek, Pk > PNa, due to leak K+ channels
Local membrane is brought to threshold voltage by a depolarizing stimulus
Current through opening voltage-gated Na+ channels rapidly depolarizes the membrane, causing more Na+ channels to open
Inactivation of Na+ channels and delayed opening of voltage-gated K+ channels halt membrane depolarization
Outward current through open voltage-gated K+ channels repolarizes the membrane back to a negative potential
Persistent current through slowly closing voltage-gated K+ channels hyperpolarizes membrane toward Ek; Na+ channels return from inactivated state to closed state (without opening)
Closure of voltage-gated K+ channels returns the membrane potential to its resting value
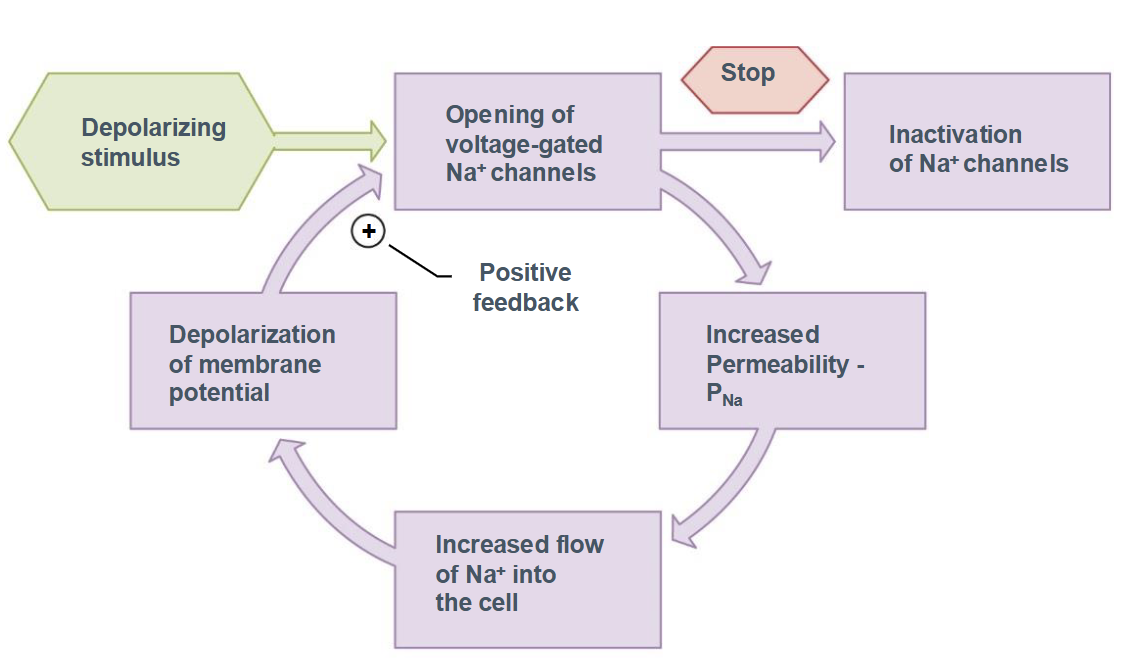
Function of Sodium Ion Channels
Depolarizing stimulus
Opening of voltage-gated Na+ channels
Increased permeability of PNa
Increased flow of Na+ into the cell
Depolarization of the membrane potential leads to positive feedback loop
Eventual inactivation of Na+ channels
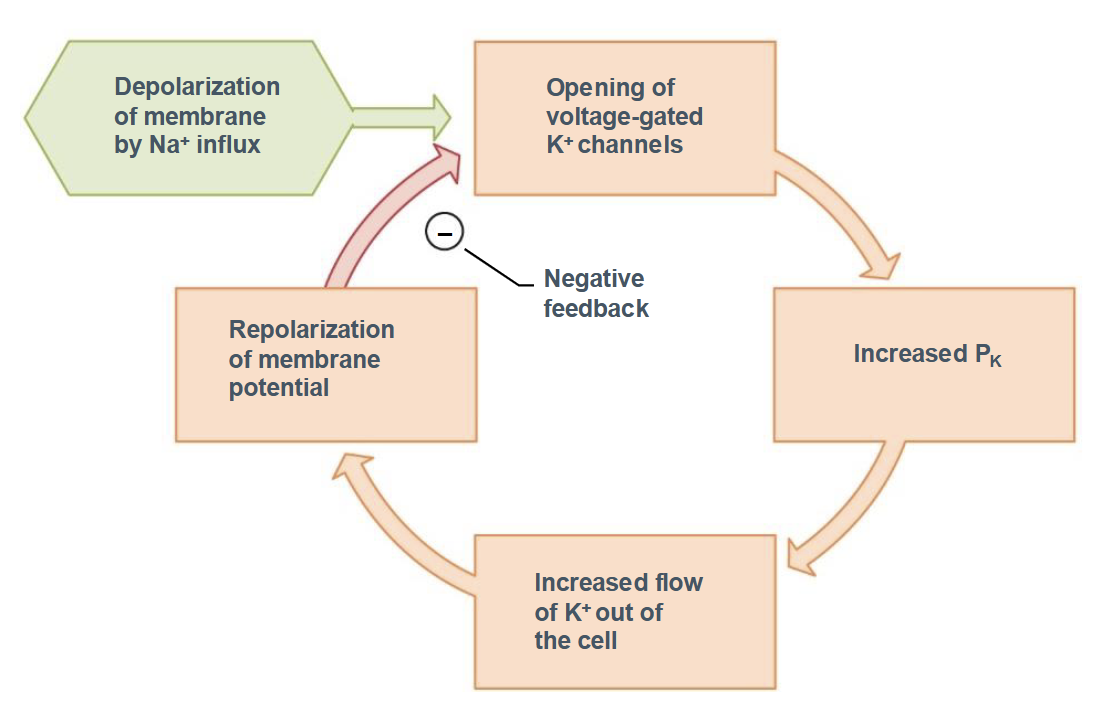
Function of Potassium Ion Channels
Depolarization of the membrane by Na+ influx
Opening of voltage-gated K+ channels
Increased PK
Increased flow of K+ out of the cell
Repolarization of the membrane potential leads to negative feedback loop
Refractory Period
The short time after a cell (usually a neuron or muscle cell) fires an action potential during which it cannot fire again normally. It can be:
Absolute
Relative
Absolute refractory period
No second action potential is possible, no matter how strong the stimulus.
Happens because voltage-gated Na⁺ channels are inactivated.
This guarantees that action potentials move in one direction only down the membrane
Relative refractory period
A second action potential is possible, but only with a stronger-than-normal stimulus
Occurs while K⁺ channels are still open and the membrane is hyperpolarized
Some Na+ channels have reset, but some are still inactivated
Action potential propagation
the movement of the action potential along the membrane of an axon or muscle cell
The signal does not weaken as it travels (it’s all-or-none), because the action potential is regenerated at each segment of membrane
Saltatory conduction
Myelin insulates the axon
Action potentials occur only at Nodes of Ranvier
Signal “jumps” node to node
Much faster and more energy efficient
Nodes of Ranvier
small, unmyelinated gaps between adjacent myelin sheath segments along a myelinated axon
Rich in voltage-gated Na⁺ (and K⁺) channels
Site where action potentials are regenerated
Enable saltatory conduction (the signal “jumps” from node to node)
Why might there be two different kinds of chemical synapses?
excitatory
inhibitory
the nervous system needs both acceleration and braking to function properly
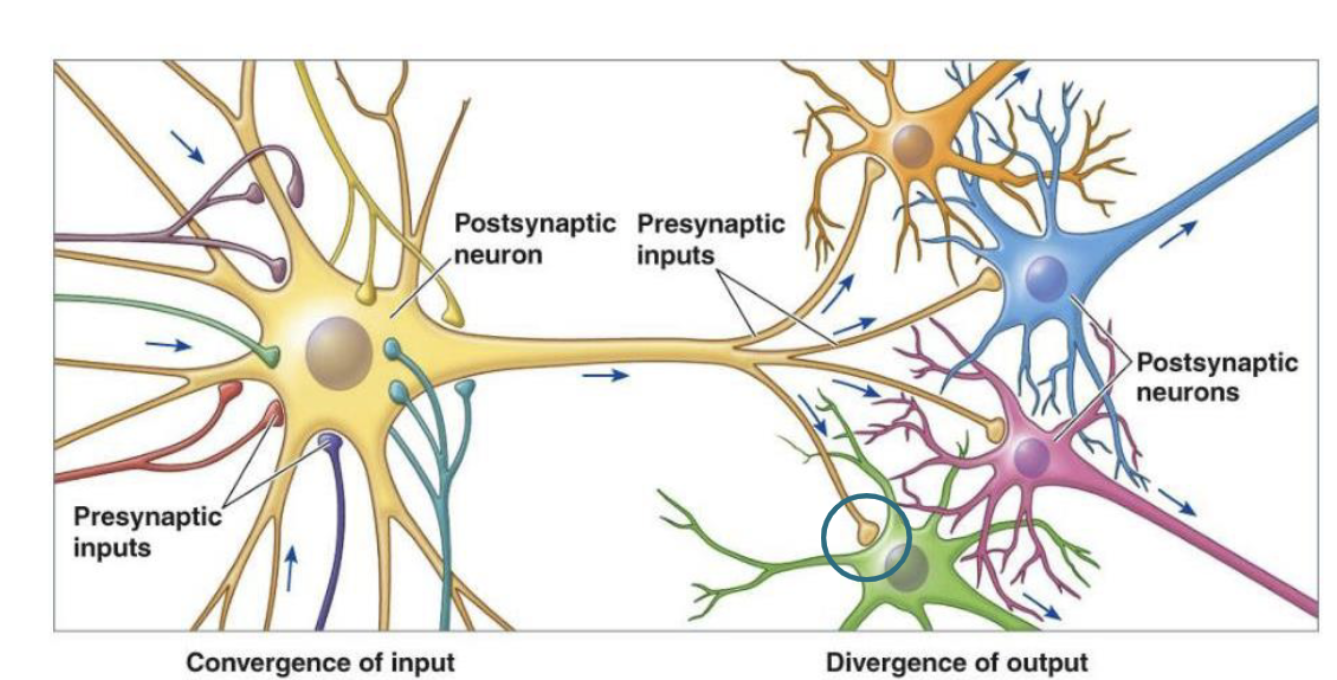
Convergence of input
one cell is influenced by many others
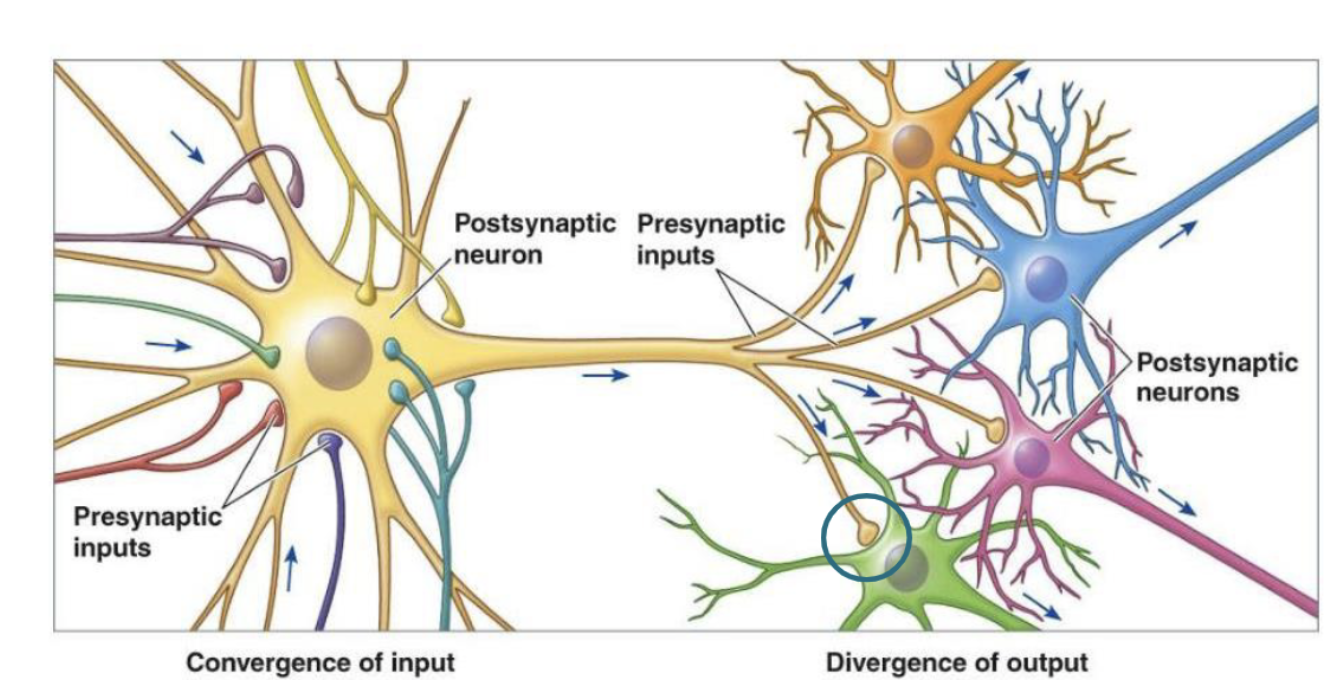
Divergence of input
one cell influences many others
Steps of Activating a Presynaptic Cell
Action potential reaches axon terminal
Voltage-gated Ca2+ channels open
Calcium enters the axon terminal
Neurotransmitters are released and diffuse into the synaptic cleft
Neurotransmitters bind to their postsynaptic receptors
Neurotransmitters are removed from the synaptic cleft
Vesicle docking
the step in synaptic transmission where neurotransmitter-filled synaptic vesicles attach to the presynaptic membrane, positioning them for release
Occurs at the active zone of the presynaptic terminal
Mediated by SNARE proteins
Puts vesicles in a ready-to-release state
After docking, an incoming action potential opens voltage-gated Ca²⁺ channels → Ca²⁺ enters → triggers vesicle fusion and exocytosis of neurotransmitter.
Synaptotagmin Function
the calcium sensor that triggers neurotransmitter release at chemical synapses
An action potential reaches the presynaptic terminal
Voltage-gated Ca²⁺ channels open → Ca²⁺ enters
Ca²⁺ binds to synaptotagmin on the synaptic vesicle
Synaptotagmin changes shape and interacts with SNARE proteins
This causes vesicle fusion with the presynaptic membrane and exocytosis of neurotransmitter
SNARE Proteins Function
Mediate synaptic vesicle docking and fusion by forming a complex that pulls the vesicle and presynaptic membranes together, enabling neurotransmitter release
These proteins zip together, pulling the vesicle tightly against the membrane
Provide the mechanical force needed for membrane fusion
Ensure vesicles fuse at the correct location (active zone)
Allow rapid, Ca²⁺-triggered exocytosis once synaptotagmin is activated
Partial fusion
The vesicle briefly contacts the presynaptic membrane
A small fusion pore opens
Some neurotransmitter is released
Vesicle then detaches and is recycled
Fast and energy-efficient, allows rapid, repeated signaling
Complete fusion
The vesicle fully merges with the presynaptic membrane
All neurotransmitter is released
Vesicle membrane becomes part of the presynaptic membrane
Requires endocytosis to retrieve membrane
ensures maximal transmitter release when needed
Ionotropic receptors
Ligand-gated ion channels that open directly upon neurotransmitter binding, allowing rapid ion flow and fast synaptic signaling
Neurotransmitter binding causes an immediate conformational change
Ions (Na⁺, K⁺, Ca²⁺, or Cl⁻) flow across the membrane
Produce fast, short-lasting postsynaptic responses
Generate EPSPs (depolarization) or IPSPs (hyperpolarization)
Examples: Nicotinic ACh receptors (neuromuscular junction), GABA
Metabotropic receptors
G-protein–coupled receptors that indirectly modulate ion channels or intracellular signaling pathways, producing slower but longer-lasting effects on the postsynaptic cell
Neurotransmitter binding → receptor activates a G-protein
G-protein can:
Open/close ion channels indirectly
Activate second messenger pathways (cAMP, IP₃, DAG)
Effects are slower but longer-lasting than ionotropic responses
Often produce modulatory effects rather than direct depolarization or hyperpolarization
Examples: Muscarinic ACh receptors
Neurotransmitter Removal from Synapse
Enzymatic degradation → Ex. ACh broken down by acetylcholinesterase
Reuptake into presynaptic neuron → transporter proteins pump neurotransmitters back into the presynaptic terminal, they can be repackaged into vesicles or degraded by enzymes inside the neuron
Diffusion away from the synapse → then they can be taken up by nearby glial cells or diluted in ECF
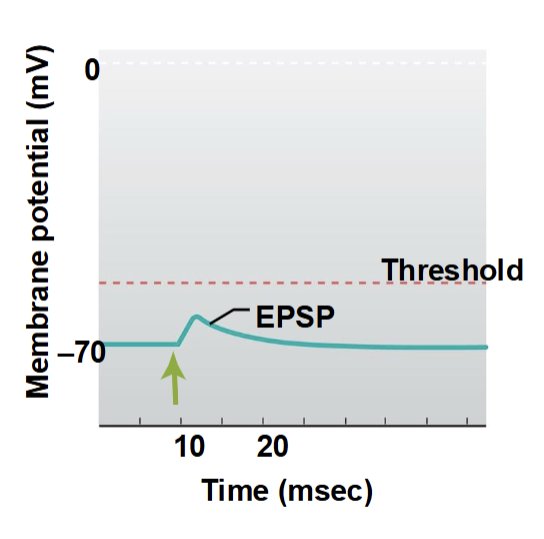
Excitatory Postsynaptic Potentials (EPSPs)
depolarizing postsynaptic potential caused by excitatory neurotransmitter binding, which increases the likelihood of an action potential
Caused by opening of ligand-gated ion channels (usually Na⁺ or Ca²⁺)
Results in positive charge entering the postsynaptic cell → membrane potential moves closer to threshold
Can summate (spatially or temporally) to trigger an action potential at the axon hillock
Example: ACh at nicotinic receptors
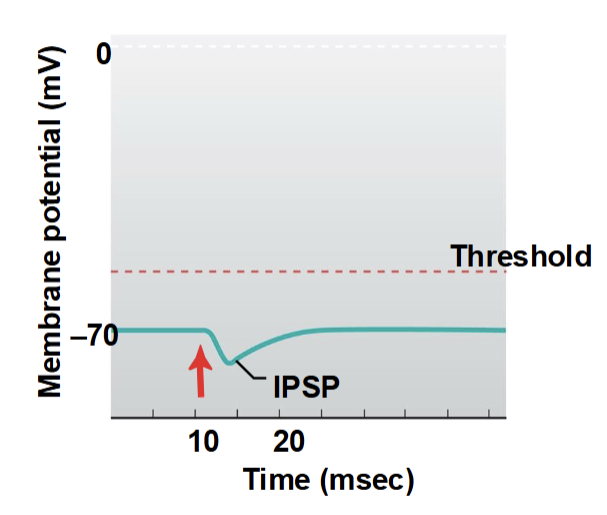
Inhibitory Postsynaptic Potentials (IPSPs)
a hyperpolarizing postsynaptic potential caused by inhibitory neurotransmitters, reducing the likelihood of an action potential
Typically caused by Cl⁻ influx or K⁺ efflux through ligand-gated channels
Temporal Summation
the additive effect of multiple postsynaptic potentials occurring in quick succession at a single synapse
Multiple EPSPs (or IPSPs) occur in rapid succession at the same synapse, and their effects add together
If the summed depolarization reaches threshold → action potential fires
Spatial Summation
the combined effect of postsynaptic potentials from multiple synapses occurring simultaneously on a neuron
The combined effect can bring the neuron to threshold or inhibit firing
Autoreceptors
presynaptic receptors that monitor and regulate the neuron’s own neurotransmitter release
Regulate neurotransmitter release, often providing negative feedback to prevent excessive release
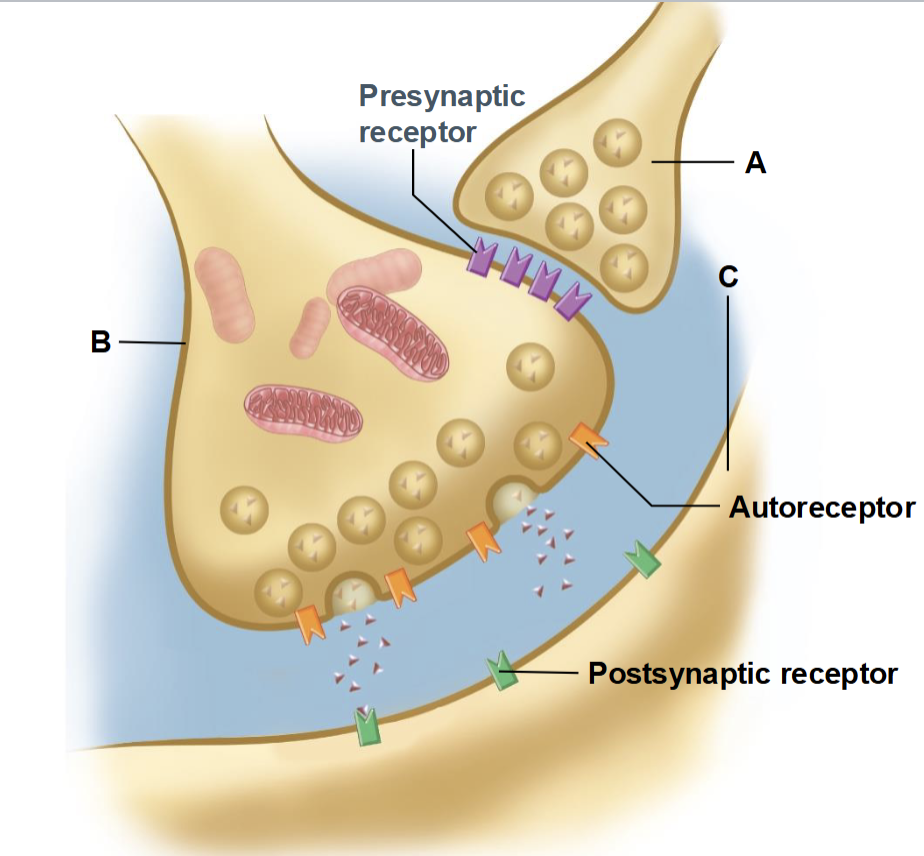
Axo-axonic neurons
neurons that synapse onto the axon of another neuron to regulate its neurotransmitter release
Can modulate neurotransmitter release by either enhancing or inhibiting it (presynaptic inhibition or facilitation)