Ap Biology review 1
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
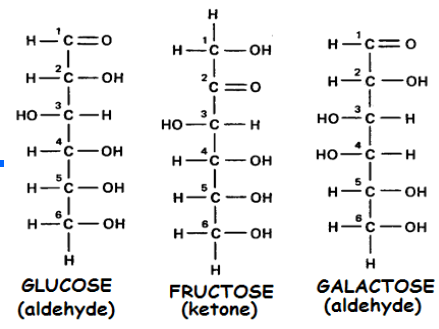
The sugars shown are examples of which type of isomer?
Structal isomers
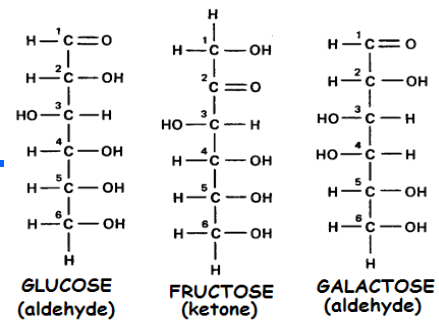
Fructose is an example of a(n)
ketone?sugar
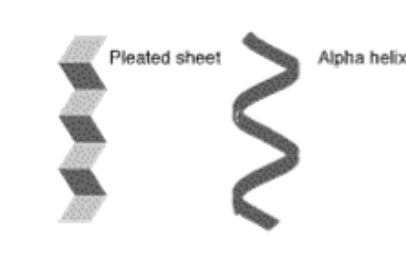
These shapes are examples of which level of protein structure
Secondary
Which kind of bond is responsible for the emergent properties of water such as cohesion, adhesion, and surface tension?
Hydrogen bonds
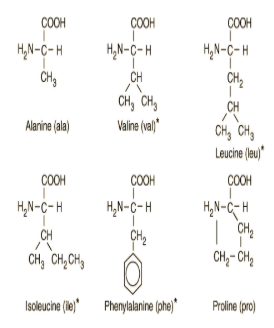
This molecule is an example of_____ which type of building _____ block used to make macromolecules?
Amino acids

Flask A is_____times_____ acdic than flask B
1000 more
Nucleotides are to______________ as___________ are to proteins.
nucleic acids and amino acids
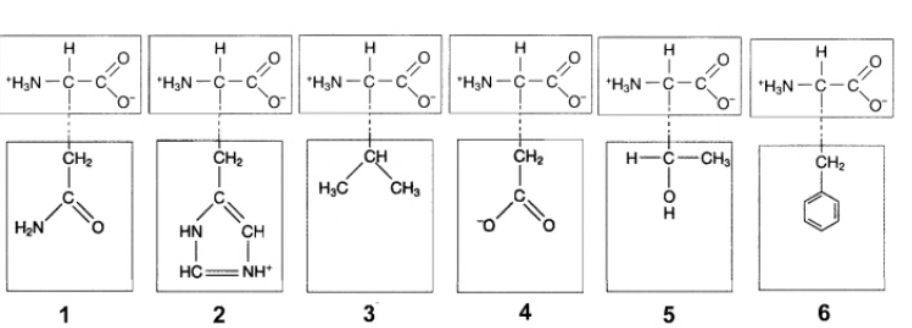
In the figure above, ionic attractions would form between the R groups of which amino acids?
2 and 4
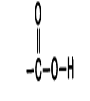
Identify this functional group
Carboxyl Group
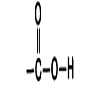
How does adding this group to a molecule change its characteristics?
More polar hydrophilic; weakly acidic; can become negatively charged if H is lost
=
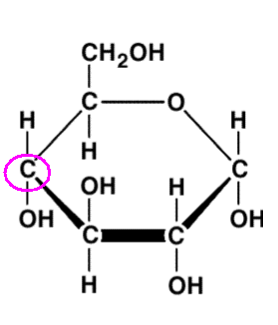
Use the numbering system you learned about to number the circled carbon.
C4
Tell a polysaccharide that could be built using this molecule as a subunit.
glycogen or starch
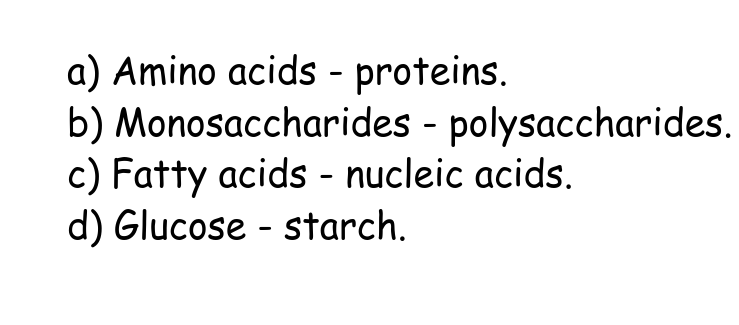
Which monomer is incorrectly matched with its corresponding polymer?
C Fatty acids - nucleic acids.
Tell one way DNA and RNA are different.
DNA is double stranded RNA is single stranded
Name the subunits used to make a triacylglycerol.
glycerol + 3 fatty acids
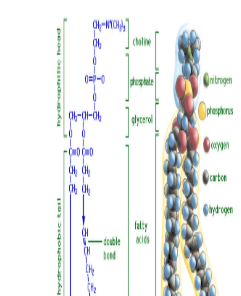
Which part of a phospholipid is polar, the head or the tails?
head is polar
Chitin, cellulose, amylopectin, amylose, and glycogen are all examples of
polysaccharides
Nitrogen bases with 2 rings like adenine and guanine are called
Purine
Animals store their sugar as
glycogen
Explain how hydrolysis and dehydration synthesis play a role in the charging and release of energy from ATP, the rechargeable power molecule used by cells for energy.
dehydration synthesis adds the phosphate to charge it; hydrolysis removes it releasing energy
Tell how the function of polysaccharides made from α-glucose and β-glucose are different.
a-glucose makes polysaccharides for energy storage (starch & glycogen); β-glucose makes structural polysaccharides (cellulose & chitin)
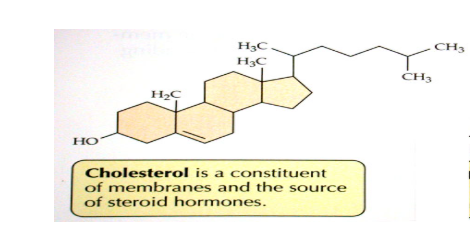
Lipids with this basic structure (3 rings and a dog house) are called
steroids
______ is the attraction of water molecules to a surface.
adhesion
In an experiment 100 plants are grown in identical pots, watered the same amount, and given the same amount of light. A fan is placed to blow a slight breeze on 50 of the plants. The other 50 are grown without a fan. Transpiration rate is measured and recorded. Identify the dependent and independent variables in this experiment.
independent variable – breeze; dependent variable-transpiration rate
Fatty acids with at least one double bond are called u
Unsaturated
Tell how a peptide and protein are different
polypeptide = straight chain sequence; when it folds into its 3D shape = protein
Name a functional group that would make a molecule more hydrophobic.
methyl
____bonds are covalent bonds that hold amino acids together in a polypeptide chain.
Peptide
Polar molecules are ________________
hydrophilic
Name the parts of a nucleotide subunit.
sugar + phosphate + nitrogen base
Which of these end up in the backbone (sides of the ladder) in a DNA molecule?
sugars and phosphates
Carbohydrates made with only TWO sugars
joined together like sucrose or lactose are called ____
disaccharide
Name a nitrogen base found in RNA but not DNA.
uracil
Tell the kind of glycosidic linkage found in
cellulose and chitin
β 1-4 linkage
What functional group does cysteine have that allows it to form disulfide bonds?
sulfhydryl group
Which fatty acid tail in this phospholipid is unsaturated?
B is unsaturated
How can you tell its unsaturated?
Double bonds put “kinks” in fatty acid carbon chains.
The figure at the right shows the calories of heat energy required to convert a gram of water from solid to liquid state, and then again from liquied to a gaseous state. This graph predicts which of the following properties of water that would
affect plant survival
a) Plant leaves doing transpiration are cooled down on hot days.
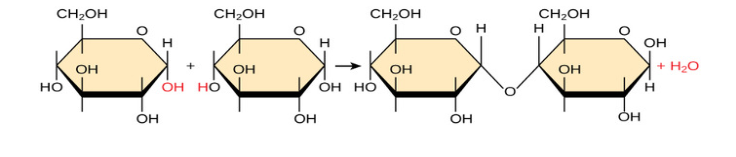
Name this chemical reaction.
dehydration synthesis