biology - nucleic acids
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
71 Terms
what are nucleic acids?
ribonucleic acid (RNA)
deoxyribonucleic acid (DNA)
features of DNA:
double helix
carries genetic information
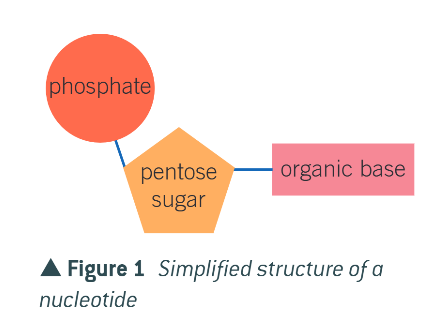
made up of nucleotides with just 3 basic components
what are the 3 components of nucleotides?
pentose sugar (5 carbons)
phosphate group
nitrogen containing organic base (cytosine, thymine, uracil, guanine, adenine
how are these 3 components joined?
condensation reactions
what does this form?
a mononucleotide
how might 2 mononucleotides be joined to make a dinucleotide?
condensation reaction between deoxyribose sugar and phosphate group
what is the bond that forms between these?
phosphodiester bond
what does the continued linking of mononucleotides form?
a polynucleotide
do other biologically important molecules besides DNA and RNA have nucleotides?
yes
symbols for simplicity to show components of nucleotides:
what is the structure of RNA?
polymer of nucleotides
single polynucleotide chain in which then pentose sugar is always ribose
what are the organic bases of RNA?
adenine, guanine, cytosine and uracil
3 types of RNA?
transfers genetic info from DNA to the ribosomes
ribosomes made up of proteins and another type of RNA
involved in protein synthesis
structure of DNA?
pentose sugar: deoxyribose
made up of 2 strands of nucleotides
strands joined by hydrogen bonds at certain bases
what are the organic bases for DNA?
adenine, thymine, guanine and cytosine
complementary base pairing? (joined by hydrogen bonds)
adenine with thymine
guanine with cytosine
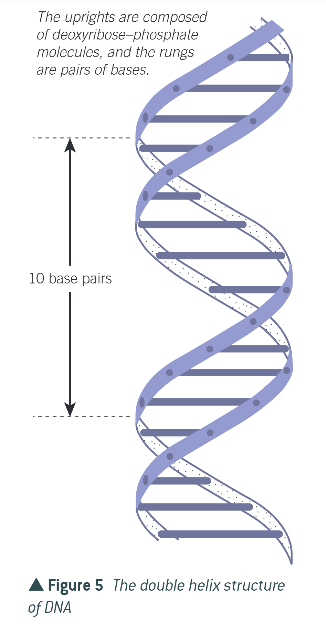
double helix structure:
2 polynucleotide chains are twisted
phosphate and deoxyribose form structural backbone of DNA molecule
why is DNA a stable molecule?
phosphodiester backbone protects the more chemically reactive organic bases in the double helix
hydrogen bonds link the bases forming bridges between the phosphodiester backbone (3 hydrogen bonds between C-G, 2 hydrogen bonds between A-T)
what is DNA responsible for?
passing genetic info from cell to cell. 3.2 bill base pairs in a typical cell, leading to great genetic variety
how is DNA adapted to carry out its functions?
stable structure which passes from gen to gen without big change. most mutations are repaired, so persistent mutations are rare
2 separate strands joined with hydrogen bonds, which allow them to separate during DNA replication and protein synthesis
carried lots of genetic info from large size
base pairs in the helical cylinder of the deoxyribose phosphate backbone which protects the genetic info from being corrupted by outside forces
base pairing leads to DNA being able to replicate and transfer info as mRNA
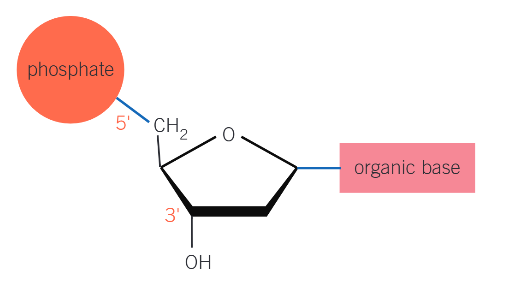
what are the important carbon atoms in a pentose sugar in the structure of DNA?
3’ and 5’ carbon atoms (5’ attached phosphate, 3’ attached hydroxyl)
what are the DNA strands?
antiparallel to one another (one runs 5’ to 3’ and the other runs 3’ to 5’
what are the 2 main stages of cell division?
nuclear division (mitosis or meiosis)
cytokinesis
what must happen before this can take place?
DNA replication, which must be precise to ensure that the cells are genetically identical
what are the 4 requirements for semi conservative replication?
the 4 types of nucleotide with their bases must be present
both strands of the DNA molecule act as a template for the attachment of these nucleotides
the enzyme polymerase
chemical energy to drive the process
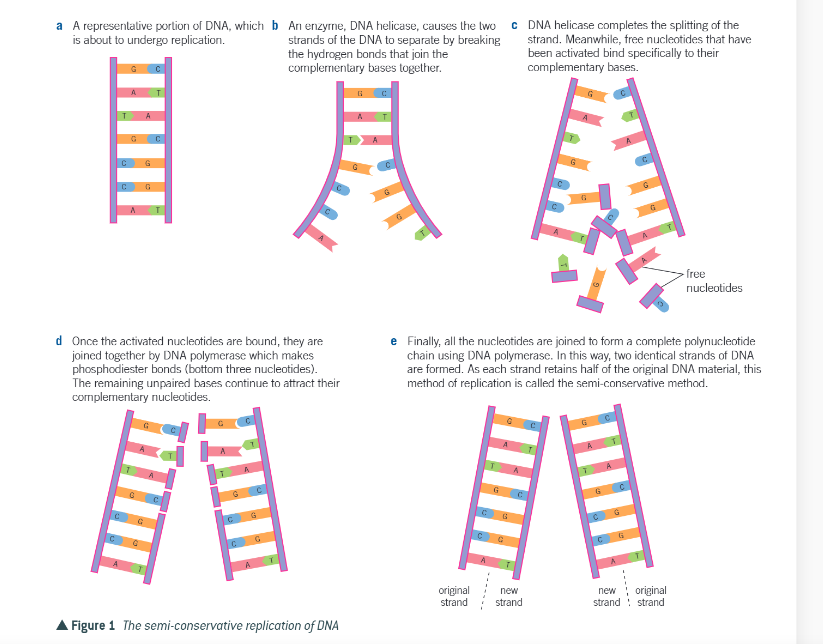
what is the process of semi conservative replication?
helicase breaks hydrogen bonds linking the base pairs of DNA
double helix separates and unwinds
each exposed polynucleotide acts as a template to free complimentary nulceotides by specific base pairing
nucleotides joined together in condensation reaction by polymerase to form the template polynucleotide
each new DNA molecule has one original DNA strand and one template
summary of the semi conservative replication of DNA
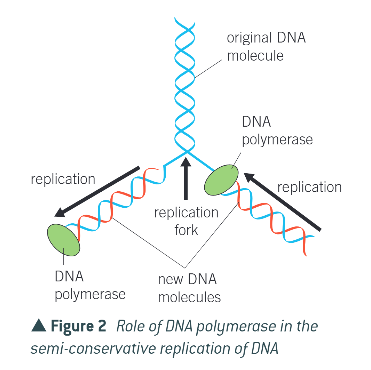
summary of DNA polymerases’ role:
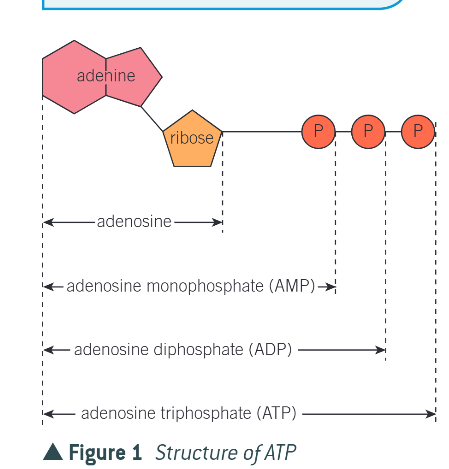
what is ATP?
adenosine triphosphate (main energy source in cells)
what is an ATP molecule?
a phosphorylated macromolecule
what are the 3 parts of ATP?
adenine - nitrogen containing organic base
ribose - pentose sugar that acts as a backbone
chain of 3 phosphate groups
is ATP a nucleotide?
yes
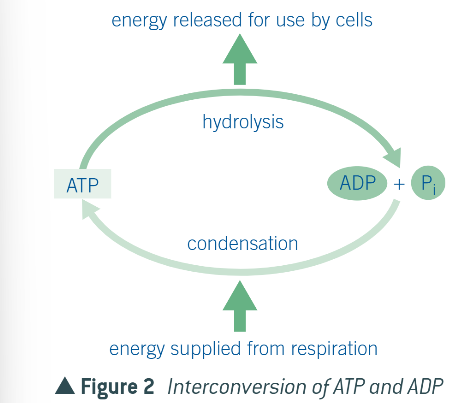
what is the issue about the bonds between the 3 phosphate groups?
they are unstable and so have a low activation energy, being broken easily
what happens when broken?
they release a considerable amount of energy

what is the reaction above known as?
hydrolysis, as it uses water to convert ATP into ADP
what is the reaction catalysed by?
ATP hydrolase
can ADP be made back into ATP?
yes, as it is reversible, via a condensation reaction and the catalyst ATP synthase
summary of the equations:
where does the synthesis of ATP from ADP involving the addition of a phosphate molecule occur?
in chlorophyll containing plant cells in photosynthesis (phosphorylation)
in plant and animal cells during respiration (oxidative phosphorylation)
in plant and animal cells when phosphate groups are transferred from donor molecule to ADP (substrate level phosphorylation)
why is ATP not a good long term energy store?
the instability of its phosphate bonds
what is ATP therefore?
an immediate energy source
why is this not an issue?
ATP is rapidly reformed from ADP and inorganic phosphate
why is ATP a better immediate energy source than glucose?
each ATP molecule releases less energy than each glucose molecule. The energy for reactions is therefore released in smaller, more manageable quantities
the hydrolysis of ATP to ADP is a single reaction that releases immediate energy. the breakdown of glucose is a long series of reactions and therefore takes longer
what has to happen because ATP cant be stored?
has to be continuously made in the mitochondria of cells that need it
what is ATP used in?
metabolic processes
movement
active transport
secretion
activation of molecules
metabolic processes:
provides energy to build up macromolecules from their basic units, eg. starch from glucose
movement:
provides energy for muscle contraction, by providing the energy for the filaments of muscle to slide past one another and shorten the overall length of a muscle fibre
active transport:
provides energy to change the shape of carrier proteins in plasma membranes, which allows molecules to be moved against a concentration gradient
secretion:
ATP is needed to form the lysosomes necessary for the secretion of cell products
activation of molecules:
the inorganic phosphate released during the hydrolysis of ATP can be used to phosphorylate other compounds to make them more reactive, therefore lowering the activation energy in enzyme catalysed reactions, eg. addition of phosphate to glucose molecules at the start of glycolysis
what is water made up of?
2 hydrogen atoms and one oxygen atom. the molecule has no overall charge, but the oxygen is slight negative, and the hydrogen atoms are slight positive
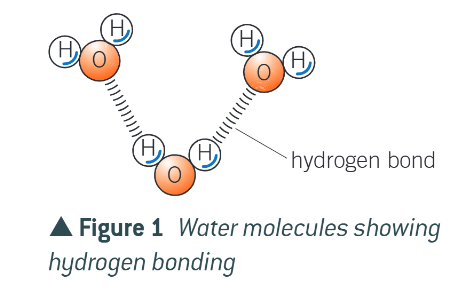
what is it therefore described as?
dipolar
how do different water molecules attract?
the positive pole of one water molecule will be attracted to the negative poles of another water molecule. this forms the weak attraction of a hydrogen bond
is the hydrogen bond weak?
yes, but there are thousands, giving overall important forces
why is the boiling point of water higher thsn expected?
takes more energy to separate them as the water molecules are bonded to each other
what does it therefore also have?
a high specific heat capacity
what does water act as?
a buffer against sudden temp variations, making the aquatic environment a temp stable one
why does water have a high latent heat of vaporisation?
hydrogen bonding between water molecules means that it requires a lot of energy to evapourate 1 gram of water
what is cohesion?
the tendency of molecules to stick together
what does waters hydrogen bonding mean?
water has large cohesive forces which allow it to be pulled up through a tube, eg. a xylem vessel
other example of waters cohesive forces:
tends to be pulled back to body of water rather than escaping at water surface. this is surface tension
why is water important to living organisms?
water is the main constituent of all organisms - 65% water is humans. it is also where life arose and where many species live
role of water in metabolism:
used to break down many complex molecules by hydrolysis, alongside being produced in condensation reactions
chemical reactions take place in an aqueous medium
water is a major raw material in photosynthesis
role of water as a solvent:
DISSOLVES:
gases, eg. oxygen and CO2
waste (ammonia and urea)
inorganic ions and small hydrophillic molecules, eg. amino acids
enzymes
other important features of water:
its evaporation cools organisms and allows for temp control
its not easily compressed and provides support
it is transparent and therefore aquatic plants can photosynthesise and light rays can penetrate jelly like fluid that fills the eye and reaches the retina
where are inorganic ions found?
in organisms where they occur in solution in the cytoplasm of cells and in body fluids as well as part of larger molecules. they may be in concentrations that range from high to low
what do inorganic ions perform?
a range of functions based on the particular ion
eg. iron ions:
found in haemoglobin and play a role in the transport of oxygen
eg. phosphate ions:
form a structural role in DNA molecules, and have a role in storing ATP molecules
eg. H+ ions:
determine the pH of solutions and functioning of enzymes