IB SL Structure 2.2: Covalent bonding
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Explain what happens with electrons in covalent bonding
In covalent bonding, atoms share electrons.
Covalent bonding (definition)
is the electrostatic attraction between the shared pair of electrons and the nuclei of the atoms making up the bond
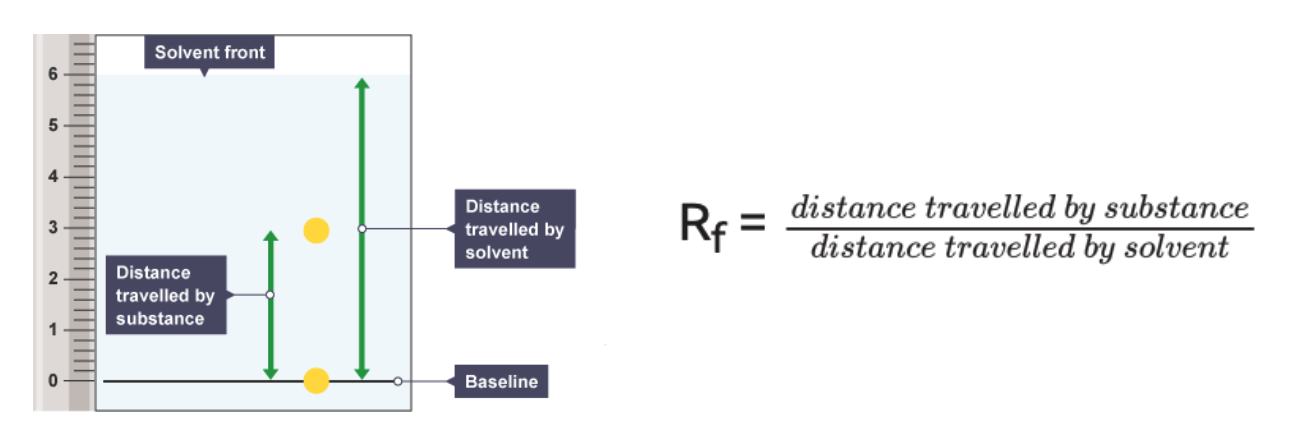
Lewis structure of CO2
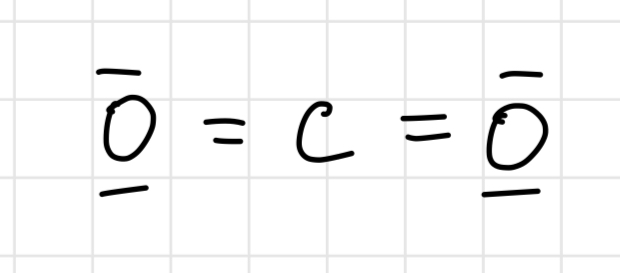
Lewis structure of PCl3
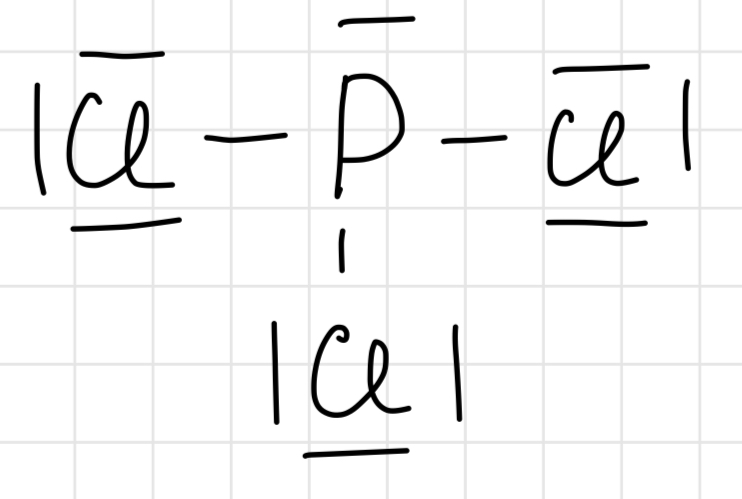
Lewis structure of NO3-
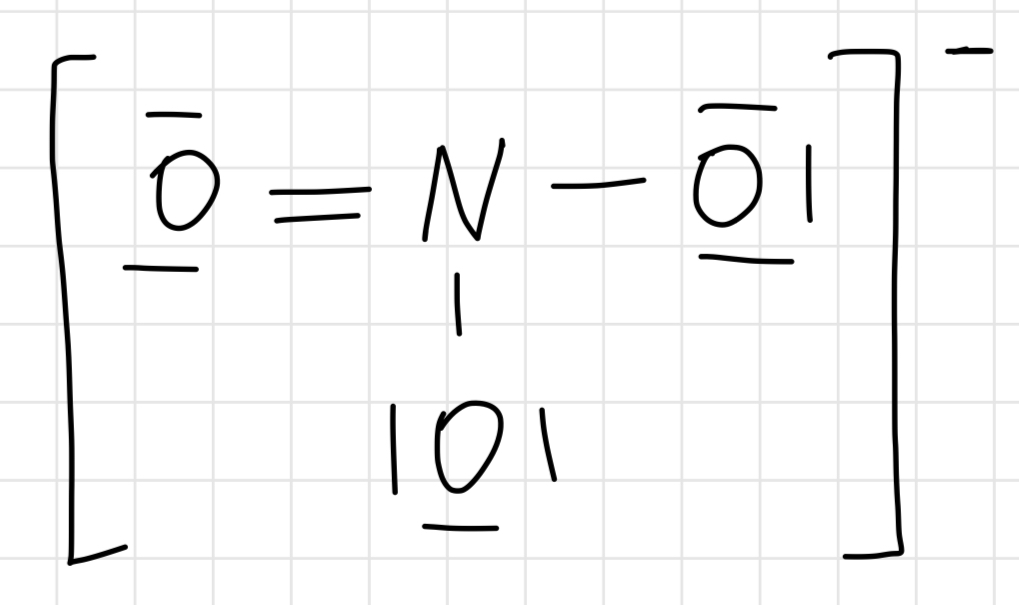
Lewis structure of BF3
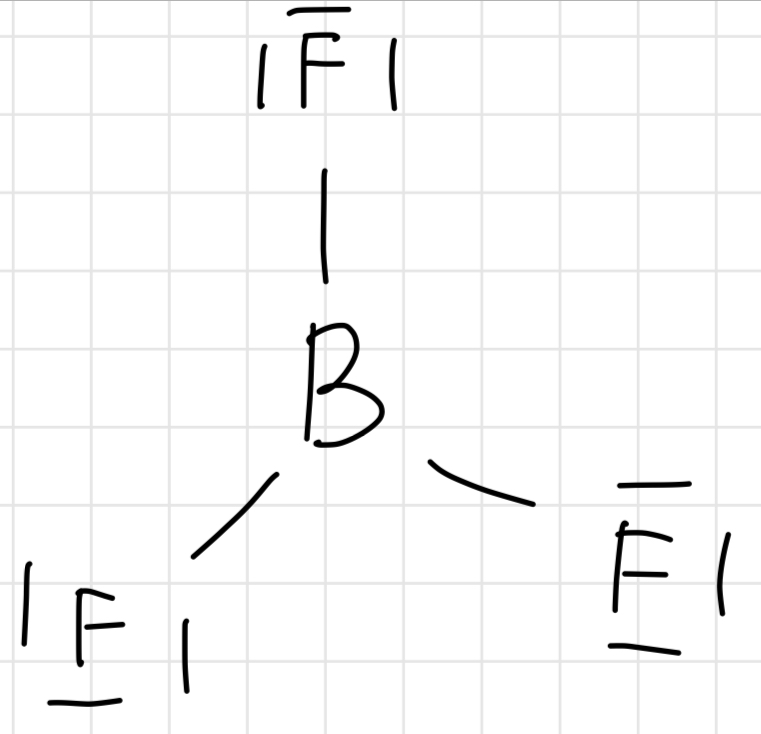
Lewis structure of H2O
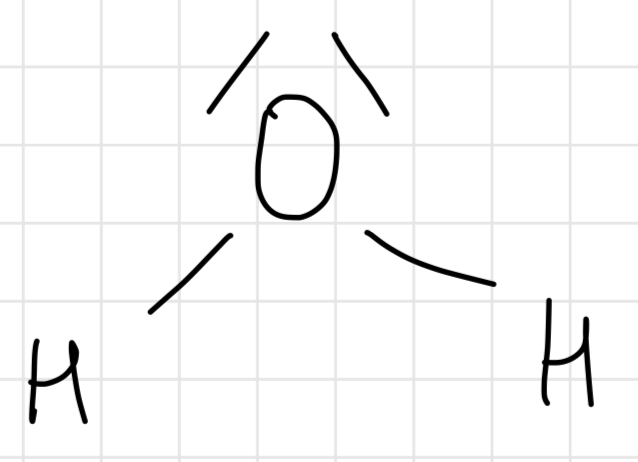
What are the steps to draw a Lewis structure
1. Identify the central atom
2. Calculate the total number of valence electron pairs
3. Connect all atoms to the central atom with single bonds (1 single bond = 1 electron pair)
4. Assign lone pairs on the side atoms, so that the octet (4 pairs) is achieved on the side atoms
5. If there are electron pairs left, assign them to the central atom
6. If the central atom does not have an octet (has less than 4 electron pairs), move lone pairs from the side atoms to form double bonds
Why are single bonds weaker than triple?
single bonds have one shared electron pair (as opposed to 3 pairs in triple bonds)
the electrostatic attraction between shared electrons and nuclei is weaker in a single bond.
Why are single bonds longer than triple?
single bonds have one shared electron pair (as opposed to 3 pairs in triple bonds)
so the electrostatic attraction between shared electrons and nuclei is weaker in a single bond, so the distance between nuclei is larger.
A dative covalent bond (coordinate bond)
is a covalent bond in which both electrons come from the same atom
State VSEPR assumptions
1. Electron domains in the valence shell of the central atom in a molecule repel each other taking position to minimise these repulsions
2. The repulsion strength:
lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair
State the shape and bond angle around an atom that has:
2 atoms bonded to the central atom
0 lone pairs
Shape: linear
Bond angle: 1800
Electron domain geometry: linear
State the shape and bond angle around an atom that has:
2 atoms bonded to the central atom
1 lone pairs
Shape: V-shaped/bent
Bond angle: 1170
Electron domain geometry: trigonal planar
State the shape, bond angle, and electron domain geometry around an atom that has:
2 atoms bonded to the central atom
2 lone pairs
Shape: V-shaped/bent
Bond angle: 104.50
Electron domain geometry: tetrahedral
State the shape and bond angle around an atom that has:
3 atoms bonded to the central atom
0 lone pairs
Shape: trigonal planar
Bond angle: 1200
Electron domain geometry: trigonal planar
State the shape and bond angle around an atom that has:
3 atoms bonded to the central atom
1 lone pairs
Shape: trigonal pyramidal
Bond angle: 1070
Electron domain geometry: tetrahedral
State the shape and bond angle around an atom that has:
4 atoms bonded to the central atom
0 lone pairs
Shape: tetrahedral
Bond angle: 109.50
Electron domain geometry: tetrahedral
What are the conditions for a molecule to be polar?
A molecule is polar when BOTH conditions are true:
The molecule has polar bonds
The molecule has such a shape that the dipoles do not cancel each other out.
What are 2 structures that covalent substances can form?
Simple molecules
Network (giant) covalent
Explain why CO₂ has a low melting point
CO₂ is covalent
CO₂ has a simple molecular structure
Weak intermolecular forces need to be broken
This requires little energy
Explain why diamond has a high melting point
Diamond has a giant (network) covalent lattice
A lot of strong covalent bonds must be be broken
This requires a lot of energy
Describe the structure of graphite
Giant (network) covalent
Each C atom is bonded to 3 other C atoms by covalent bonds.
Delocalised electrons
Forms layers
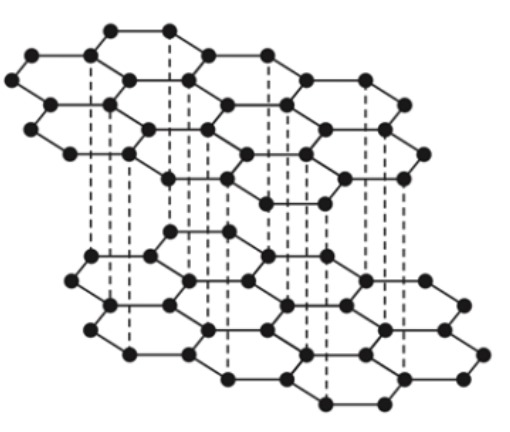
Explain why graphite is soft
No covalent bonds between layers
So layers can slide over each other
Explain why graphite conducts electricity
It has delocalised electrons that can flow and carry charge
Describe the structure of graphene
Giant (network) covalent
Each C atom is bonded to 3 other C atoms
Forms a layer (just like graphite, but it’s only 1 layer)
Delocalised electrons
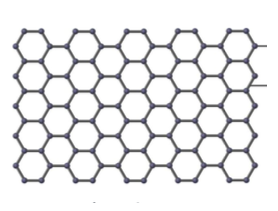
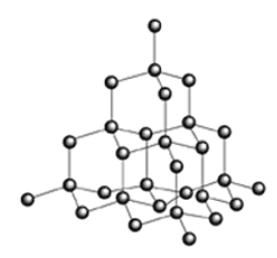
Describe the structure of diamond
Ginat (network) covalent
each C atom is bonded to 4 other C atoms by covalent bonds
No delocalised electrons
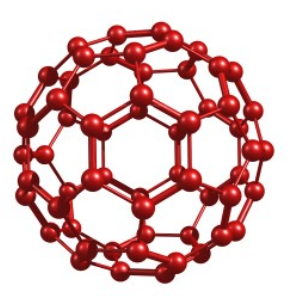
Describe the structure of fullerene
Simple covalent (molecular)
In fullerene, each C atom is bonded to 3 other C atoms by covalent bonds.
Hexagons and pentagons of C atoms.
Conducts electricity on the surface of the molecule.
Hollow inside
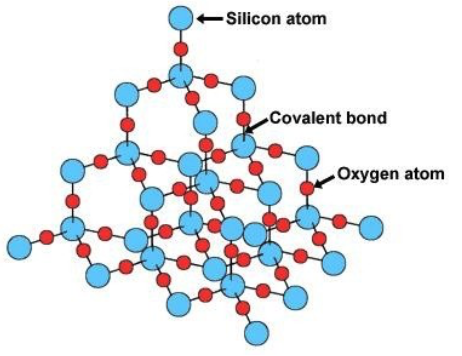
Describe the structure of silicon dioxide
Giant (network) covalent
Each Si atom is bonded to 4 O atoms
Each O atom is bonded to 2 Si atoms
No delocalised electrons
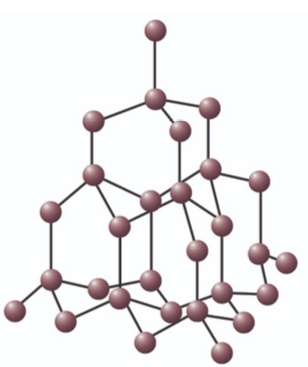
Describe the structure of silicon dioxide
Giant (network) covalent
No delocalised electrons
What are the 4 types of intermolecular forces
London
Dipole-induced dipole
Dipole-dipole
Hydrogen bonding
What IMF are between CH₄ molecules?
London
What IMF are between PCl3 molecules?
London, dipole-dipole
What IMF are between NH3 molecules?
London, dipole-dipole, H-bonding
Paper chromatography
Paper chromatography is used to separate mixtures of soluble substances. These are often coloured substances such as food colourings, inks, dyes or plant pigments.
Rf formula