ch3 - Electronic Structure and Periodic Properties of Elements
0.0(0)
0.0(0)
New
Card Sorting
1/14
Earn XP
Description and Tags
CHEM25
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
1
New cards
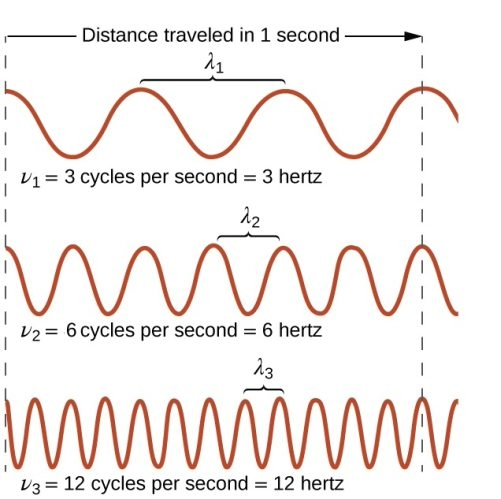
SPEED OF LIGHT
c= λν
2
New cards
C =
2.998×108m/s
3
New cards
λ =
wavelength, m
4
New cards
v (pronounced “nu”)
frequency, Hz (hertz) or s-1 (per second)
5
New cards
MHz to Hz
x106
6
New cards
Nm to m
x10-9
7
New cards
wavelength UP, frequency ____
DOWN
8
New cards
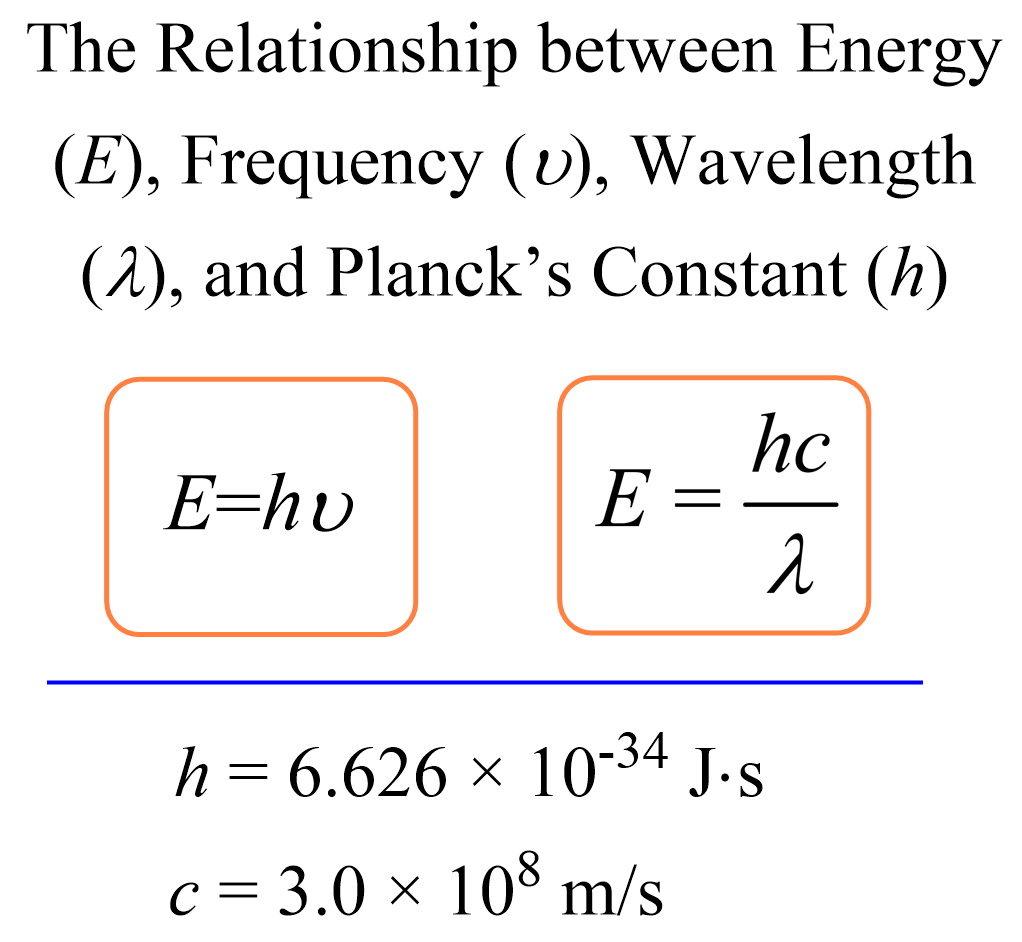
Energy Formula
E = hv or E= hc / λ (wavelength)
9
New cards
h (Plank’s constant) =
6.626 × 10-34 J
10
New cards
ENDOTHERMIC
absorb light. increase energy
11
New cards
EXOTHERMIC
release light. decrease energy
12
New cards
Difference in Energy formula from n1 to n2
E1-E2
13
New cards
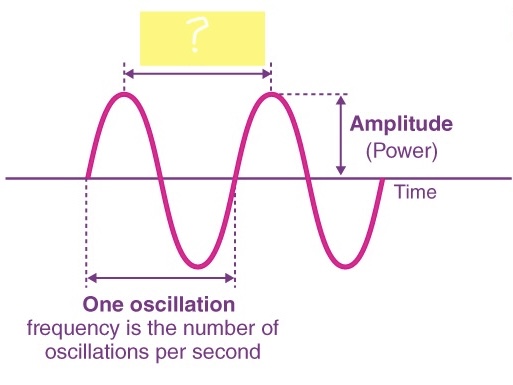
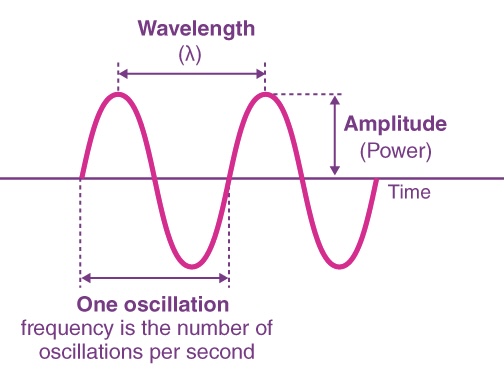
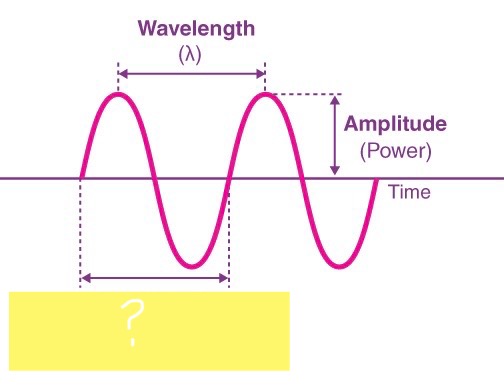
WAVELENGTH ( λ )
14
New cards
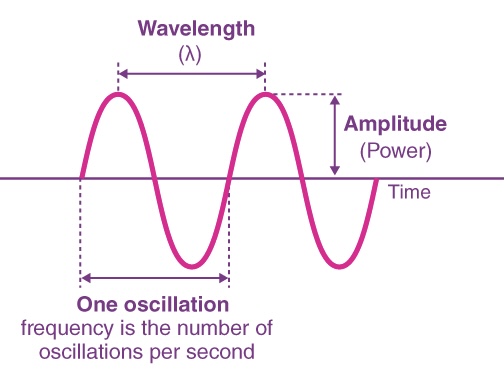
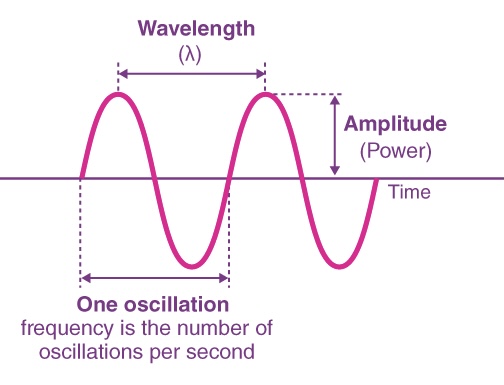
FREQUENCY ( v )
15
New cards
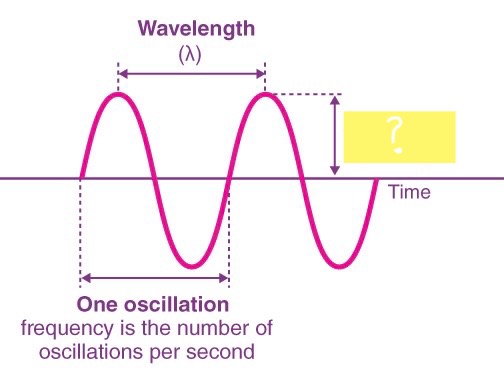
AMPLITUDE