exam 4 orgo alkynes
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
NBS/hv
allyic bornation
NaNH2 (alone or w H3O+)
forms alkyne
KOH
forms alkyne
HgSO4,H2SO4,H2O
makes keto and enol keto more favored
terminal BH3/THF,H202
makes enol then aldehyde

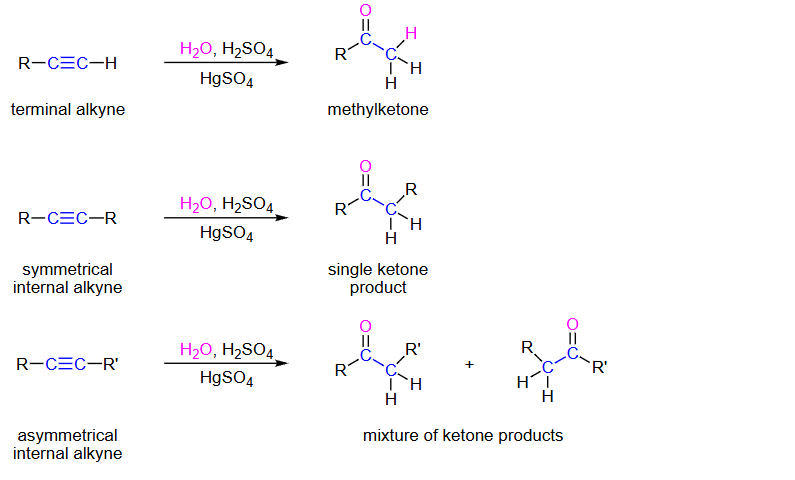
Terminal Alkyne, H2O,H2SO4,HGSO4
methyl-KETONE
Symmetrical internal alkyne H2O,H2SO4,HGSO4
single ketone
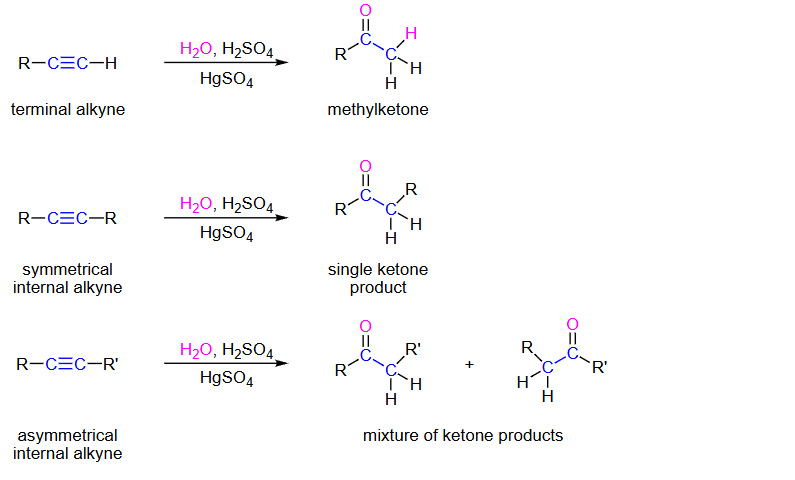
asymmetrical internal alkyne
mixture of ketones
terminal alkyne Sia2BH, 9-BBN, h2o2,oh
aldhyde
Sia2BH, 9BBN, h2o2,oh
ketone
INTERNAL ALKENE 1.BH3/THF 2.H202 H20 NAOH
ketone
H3O+/HGSO4
Makes MIX of inverted methyl ketones
H3O+/HgSO4 Terminal alkyne
methyl ketone
LI/NH3
MAKES TRANS ALKENE
KMNO4, O3
OXIDATIVE CLEAVAGE
NANH2,NH3 + X-Br
adds x-br to chain, in thf adds extra carbon
x2
adds E if eq
HBR
adds z
x2 + UV
one x replaces a H on most subed carbon
HX + ALCHOL
replaces X w OH
PBr3 + SOCl2 + alchol 1* or 2*
Pbr replaces oh 1* w br, socl 1* replaces oh w cl
E2
uses nuc to kick b C proton adjacent to LG out and kick out LG, forming a double bond- concerted
E1
LG leaves, CC forms, shifts take place, db is formed towards most subbed C
Sn2
NUC attacks adjacent H from leaving group, LG bond goes to it, leaves, nuc (from backside attack) makes new bond to carbon that hydrogen left-concerted- stero chem inverts
Sn1
LG leaves, CC forms, shifts take place, NUC attaches itself - racemic mix
cl2 hv
free radical halogenation, 3* favored over all for radical, imitation, propagation, termination
3* OH
H-x