CHE 2C - CH. 19 Transition Metals
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
69 Terms
Transition metals exhibit ____
metallic luster and high electrical and thermal conductivities
When forming ionic compounds, the cations are often ______
complex ions
Ligands
molecules or ions that behave as lewis base
Paramagnetic
electrons are unpaired
Transition metal ions show that 3d orbitals have ______ then 4s orbitals
less energy
the electron that remains when an ion is formed is from the 3d orbital
Higher OS are not seen for the first row due to 3d orbital’s _____
lower energy
increased nuclear charge, causing electrons to be difficult to remove
Metals with the most _____ are the best ________
positive potentials, reducing agents
Reducing Abilities of the first row _____
decrease left to right
The Lanthanide Contraction
2nd and 3rd row radii are the same due to poor shielding from the f-electrons thus reducing the radii in the 3rd row
Half filled and Completely Filled Shells of d-Orbital
Cr, Cu, Mo, Ag
Coordination Compounds
usually colored and paramagnetic; consists of a complex ion
Complex Ion
TM ion w/ attached ligands and the counter ion to produce a compounds with no net charge
Coordination Number
the number of bonds/ligands around the central metal atom
6 is the most common
Lewis Base
electron pair donor
Lewis Acid
electron pair acceptor
A ligand will ____ an electron pair to an empty orbital on a metal ion
donate
Monodentate
ligand can form one bond to a metal ion
Bidentate
a ligand that forms 2 bonds to a metal ion
ethylenediamine (en)
Polydentate
ligand that forms more than 2 bonds to a metal
EDTA
Fluorine
Fluoro
Chlorine
Chloro
Hydroxide
Hydroxo
Cyanide
Cyano
H2O
aqua
NH3
ammine
CO
Carbonyl
No
Nitrosyl
When naming complex compounds, how are the ligands named?
in alphabetical order
When is the latin elemental name used?
when the metal is in the anion
the compounds is bimetallic
Iron Fe
Ferrate
Copper Cu
Cuprate
Lead Pb
Plumbate
Silver Ag
Argentate
Gold Au
Aurate
Tin Sn
Stannate
Isomers
when 2 or more species have the same formula but exhibit different properties
Structural isomers
contains the same atoms but one or more bonds differ
linkage isomer
coordinate isomer
stereroisomerism
all bonds are the same but the spatial arrangement of atoms are different
Geometric Isomers
Cis (close together) and Trans (across from each other)
Optical isomers
have opposite effects on plane polarized light
Optical Activity
exhibited by molecules that have a nonsuperimposable mirror image
they are chiral
Chiral
objects that are optically active and are nonsuperimposable
Counter Ion
anion or cation needed to produce a compound w/ no net charge
Enantiomers
group of isomers that are nonsuperimposable
Linkage Isomer
same composition, different points of attachment
Coordinate Isomer
composition of the complex ion varies; switch of coordinate and counter ion
Dextrorotatory (d)
isomer that rotates the plane of light to the right
Levorotatory (l)
isomer that roates the plane of light to the left
Racemic Mixture
does not rotate the plane of the polarized light; the two opposite effects cancel
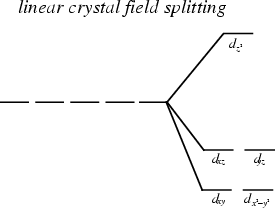
The Crystal Fields Model
color and magnetism of complex ions result from changes in the energies of the metal ion d-orbital caused by metal-ligand interactions
Definition of the Crystal Fields Model
assumes that ligands can be approximately by negative point charges and that metal-ligand bonding is entirely ionic
Strong field
large splitting - low spin
lower energy orbitals pair up before filling the upper energy
Weak Field
small splitting - high spin
electrons pair singly in each orbital before pairing up
Spetrochemical Series
list of ligands based on their abaility to produce d-orbital splitting
CN- strong field
I- weak field
The magnitude of delta ______ as charge of the metal ion increases
increases
Tetrahedral Splitting
4/9 smaller than octahedral
Due to small splitting, tetrahedrals are always what?
they are always high spin-weak field
Molecular Orbital Model
extent of overlap
relative orbital energies
Extent of Overlap in the MO Model
ligands pointing directly towards the metal are more involved
Relative Orbital Energies in the MOD Model
AOs that are close in energy will interact more strongly than those widely apart in energy
Which metals assume a square planar geometric with either 2+ or 3+ OS?
metals in Group 10 and Group 11
Whats the difference between a metal oxides and nonmetal oxides?
metal oxides: basic
nonmetal oxides: acidic
Amphoteric compounds
Al2O3 and BeO
density pattern
increases then decreases across the d-block
3rd row has a higher density due to the Lanthanide contraction
trans/mer octahedrals
achiral
4 or more of the same ligands
achiral
2 or more of the same ligands
achiral
CFM Square Planar

CFM Linear