collision theory, activation energy and catalysts
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
what is collision theory?
collision theory states that for particles to react, they have to collide with sufficient energy, and at the right orientation (direction).
what is activation energy?
the minimum amount of energy that particles require to react together.
why do particles need activation energy?
they need energy to break the bonds of the reactants, so the reaction can begin.
what does a catalyst do?
increases the rate of a chemical reaction but is not used up.
how does a catalyst increase the rate of reaction?
catalysts speed up reactions by lowering the activation energy required for a reaction to occur.
they do this by giving an alternative route (reaction pathway) that requires less energy.
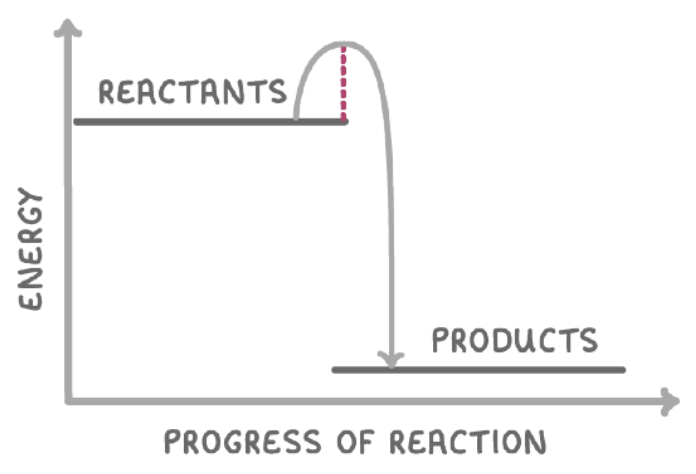
how would the above reaction profile change in the presence of a catalyst?
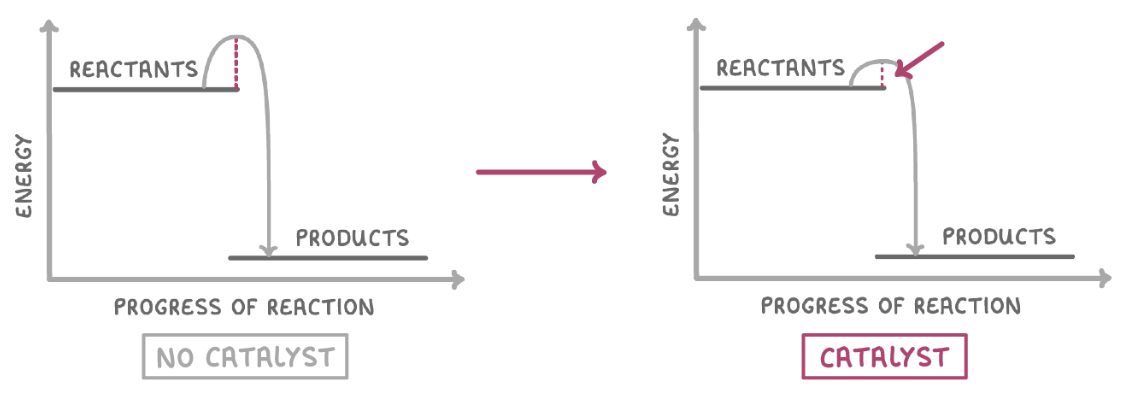
the activation energy would be lower in the presence of a catalyst.