Acidic Functional Groups and Drug Hydrolysis Products
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Common acidic functional groups in drugs
1. Carboxylic Acid
2. β-Dicarbonyl
3. Imide
4. Sulfonamide
5. Sulfonylurea
6. Sulfonic Acid
7. Phosphonic Acid
8. Phenol (with electron-withdrawing groups to make it acidic)
Percentage ionization formula for HA acids
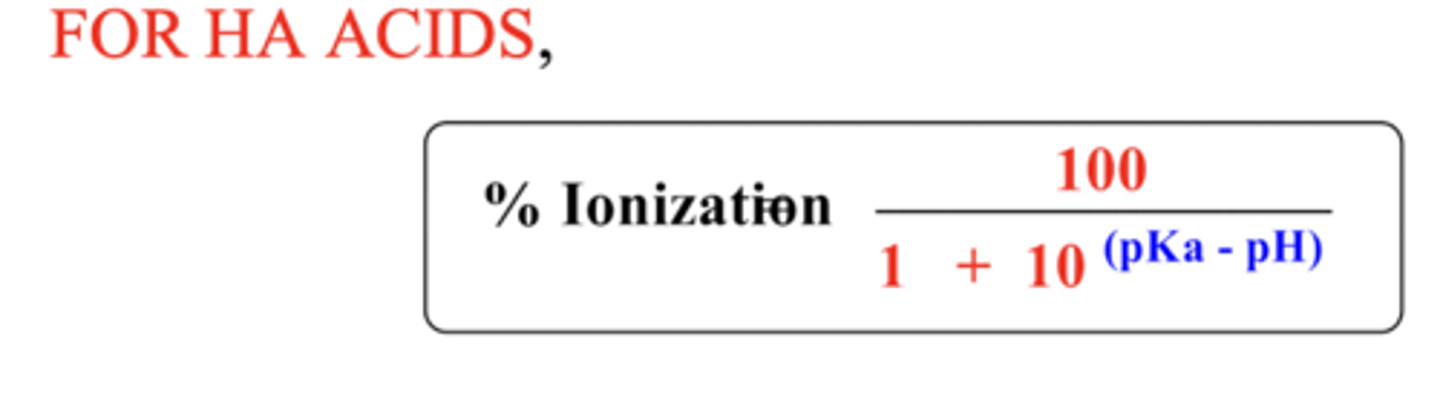
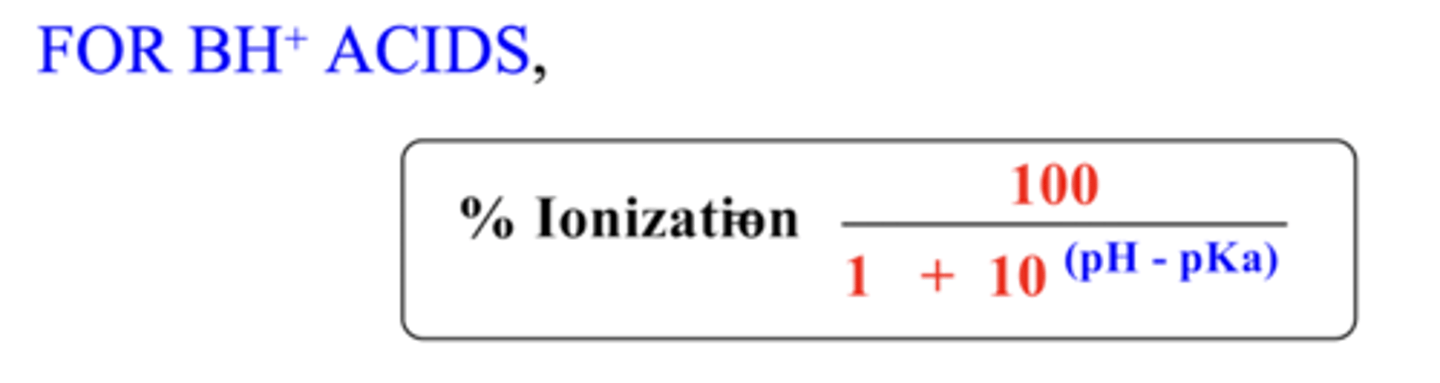
Percentage ionization formula for BH+ acids (bases)
Stronger acid in drug molecules
The presence of electron-withdrawing groups (EWGs) makes phenol a stronger acid.
Is a drug basic?
1. Nitrogen atom?
2. is nitrogen is a quaternary atom?(If yes, it is not basic.)
3. nitrogen is directly attached to a strong electron-withdrawing group (EWG) like SO₂ or C=O. If yes, the nitrogen is not basic.
4. If the nitrogen is not attached to a strong EWG, then the nitrogen is considered basic.
Product of hydrolysis of an ester functional group
carboxylic acid and an alcohol.
Product of hydrolysis of an amide functional group
carboxylic acid and an amine (or ammonia, depending on the specific amide).
Product of hydrolysis of a thioester functional group
carboxylic acid and a thiol.
Product of hydrolysis of a phosphate ester functional group
phosphate and an alcohol.
Product of hydrolysis of a lactone (cyclic ester)
Hydrolysis of a lactone yields a hydroxy acid (a molecule containing both hydroxyl and carboxylic acid groups).
Product of hydrolysis of a lactam (cyclic amide)
Hydrolysis of a lactam yields a carboxylic acid and an amine.
Product of hydrolysis of an anhydride functional group
Hydrolysis of an anhydride produces two carboxylic acids.
Product of hydrolysis of an acyl halide functional group
Hydrolysis of an acyl halide results in a carboxylic acid and a hydrogen halide (such as HCl).
Product of hydrolysis of a nitrile functional group
Hydrolysis of a nitrile yields a carboxylic acid and ammonia (or an amine intermediate in partial hydrolysis).
Product of hydrolysis of a sulfonamide functional group
Hydrolysis of a sulfonamide generally results in a sulfonic acid and an amine.