Lecture 02: Cell Chemistry and Macromolecules and Bioenergetics
1/90
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
91 Terms
The Chemical Basis of Life
Organisms are quite uniform at the molecular level → Unity of Biochemistry!
Eg. Glycolysis: takes glucose and breaks it down to generate ATP
Jacques Monod (1954) – Anything found to be true in E.Coli must also be true in Elephants.
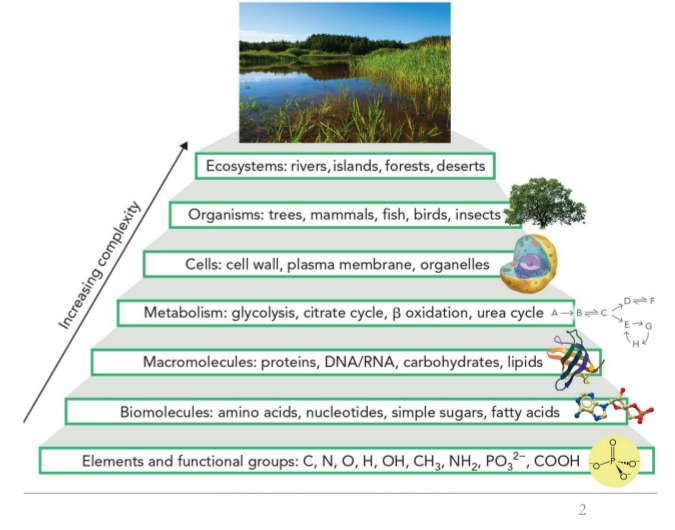
The hierarchy in increasing complexity
Main Elements in Cells
Life is dependent on chemical reactions in an aqueous environment both inside and outside the cell.
A cell is formed from carbon compounds → generally in combination with nitrogen and oxygen (elements in red) → 99% of human body composition.
Some elements are required in lower (blue, 0.9%) or trace amounts (yellow)
Atomic number on periodic table is in upper left corner
Atomic weight on periodic table is in the middle of each element
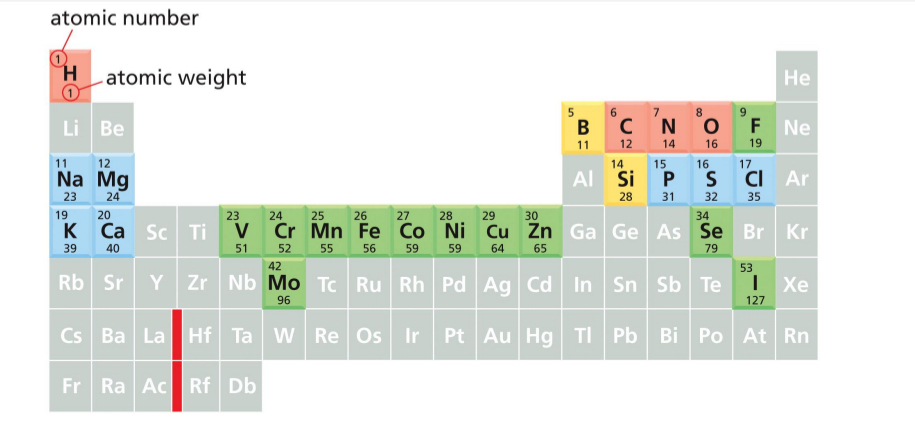
Atomic number on periodic table
is in upper left corner
Atomic weight on periodic table
is in the middle of each element
Molecules:
Two or more atoms in a definite arrangement held together by chemical bonds
Biomolecules:
Molecules made by living organisms
Mostly centred by carbon that can form single, double and triple bonds
Biomolecules centred around Carbon
Carbon binds up to 4 other atoms
Size and electronic structure is suited to generate large biological molecules
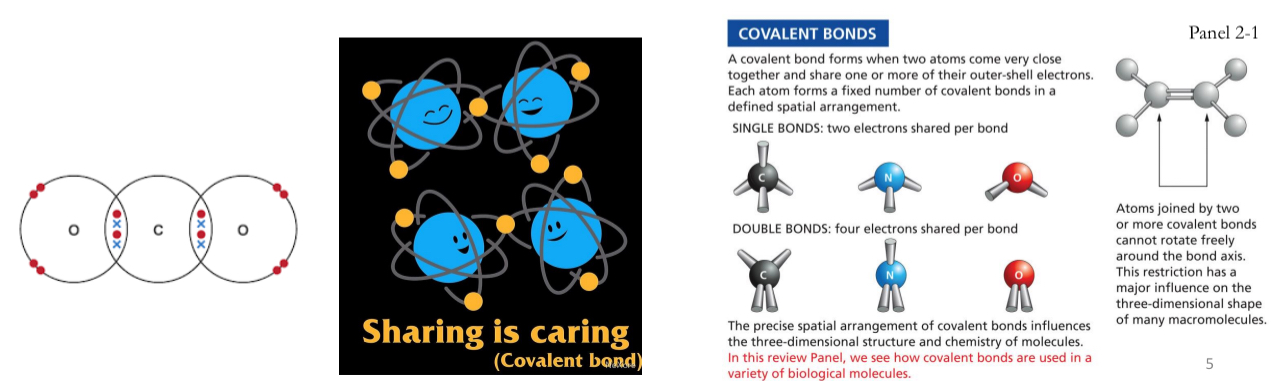
Covalent Bonds
Carbon (C), Hydrogen (H), Nitrogen (N) and Oxygen (O) can be linked together by covalent bonds to form molecules.
Atoms are most stable when their outermost electron shells are full
Can share electrons to create a stable bond → Covalent Bonds
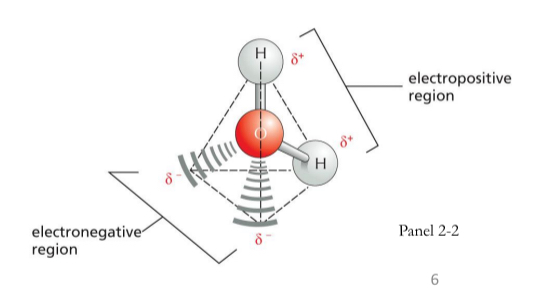
Polar Covalent Bonds
Unequal sharing of electrons
Asymmetric charge distribution:
One atom has a partial negative charge and the other atom has a partial positive charge
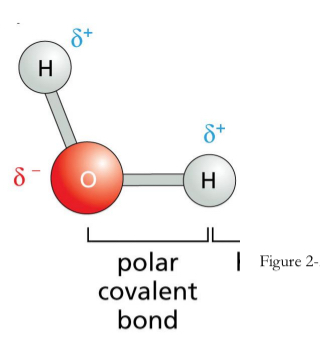
Electronegative atom:
atom with the greater attractive force is called the electronegative atom → attracts negative charge (electrons) from electropositive atom(s)
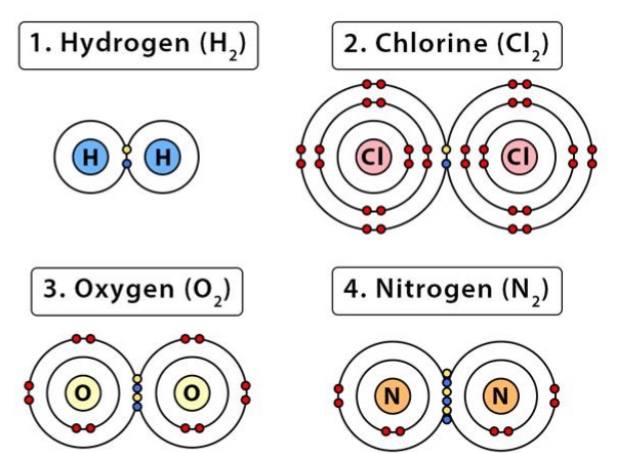
Non-Polar Covalent Bonds
Equal sharing of electrons → lack electronegative atoms
E.g. molecules made up entirely of C and H (ex Methane)
Non-Covalent Interactions
Interactions between molecules or different parts of a large biomolecule
Depend on shared attractive forces between atoms of opposite charge
Types of noncovalent interactions important in cells:
Ionic bonds
Hydrogen bonds
Van der waals attractions
Hydrophobic interactions
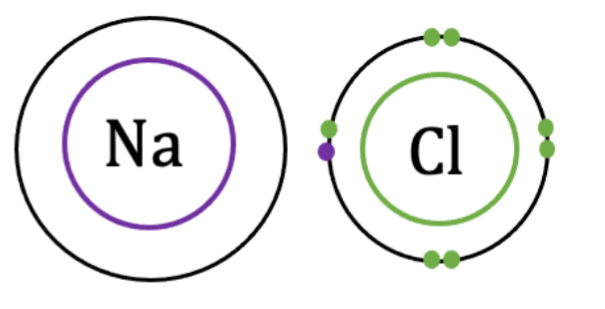
Ionic Bonds
result of electrical attraction because of opposing charges. Involves transfer of electron(s) from one atom to the other.
E.g. holds macromolecules together (DNA & protein): between positively charged nitrogen (protein) and negatively charged oxygen (DNA)
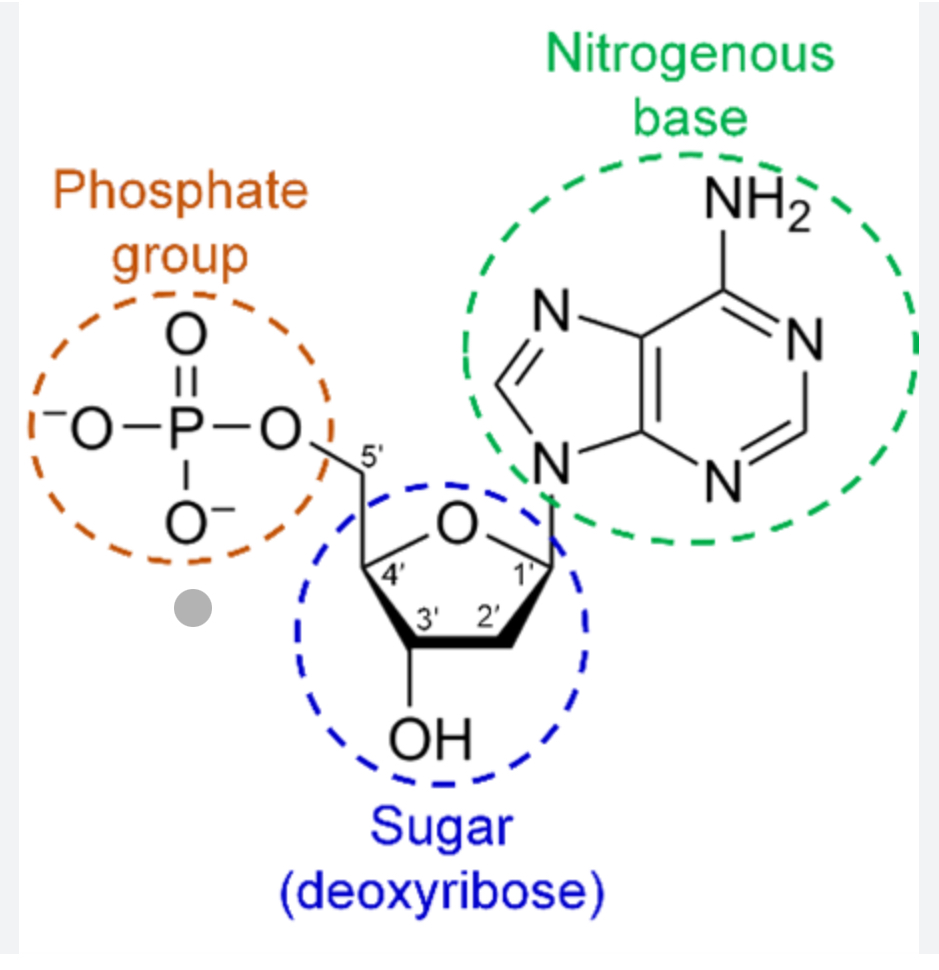
What gives DNA the negative charge
The phosphate group
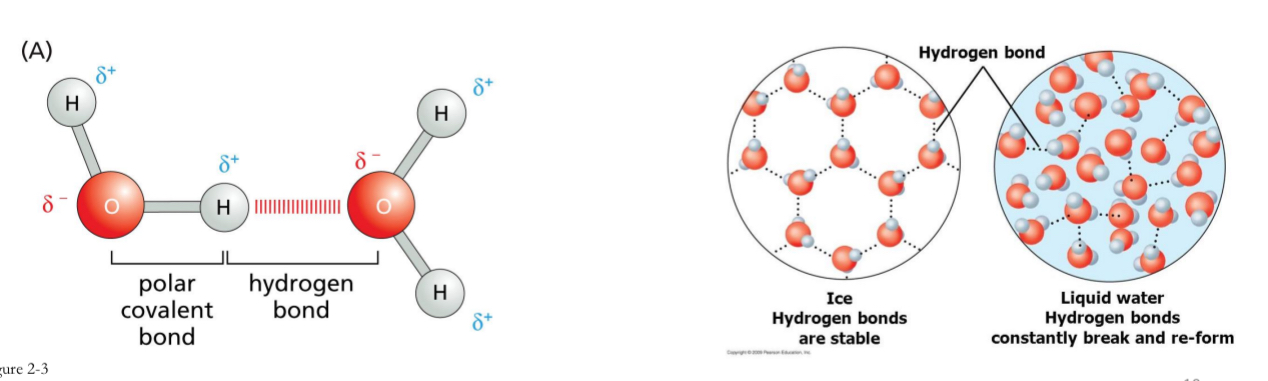
Hydrogen Bonds
weak bond, result of electrical attraction.
Keeps important biomolecular structures like DNA together
Polar molecules interact with other polar molecules, like water
Heat will easily break hydrogen bonds (denaturing of dna)
room temperature water having more energy, leading to the constant breaking and forming of hydrogen bonds, while in ice, the hydrogen bonds are largely stable due to the lower energy at colder temperatures.
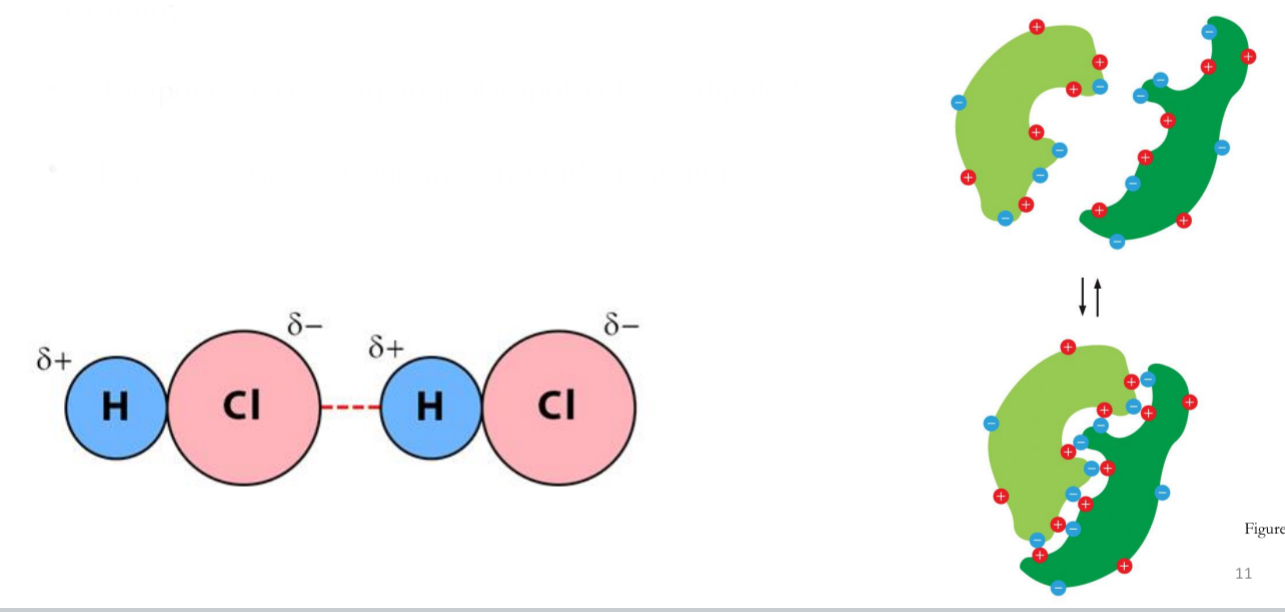
Van der Walls Attraction
Weak and nonspecific interaction between two atoms in close proximity.
Temporary charges in nonpolar molecules = ‘dipoles’
Form because electrons are constantly in motion
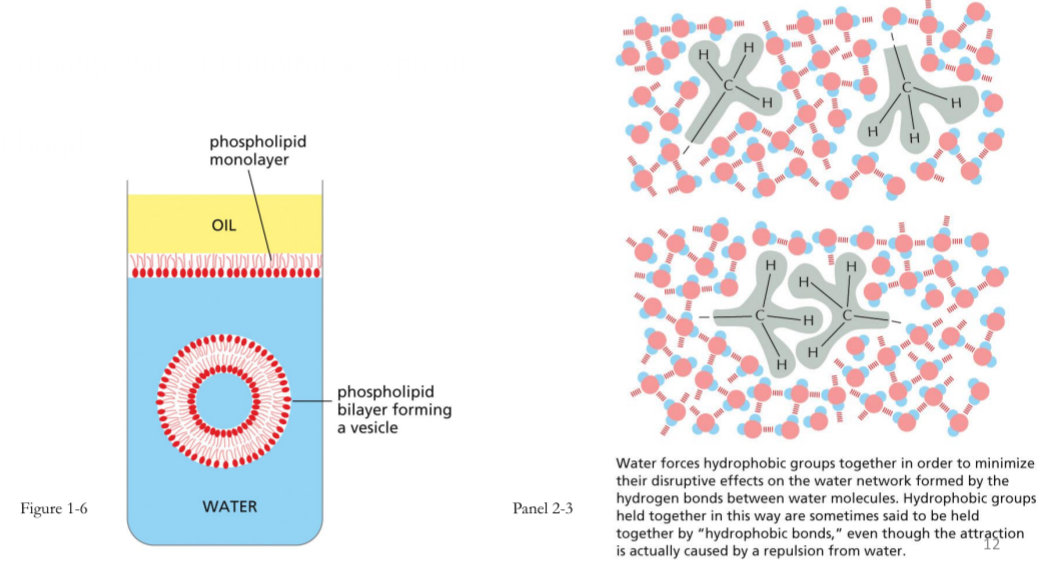
Hydrophobic Interactions
uncharged non-polar molecules do not interact with polar molecules (e.g. water)
Form clumps or aggregates to minimize exposure
Not an actual bond
Ex. Oil doesn’t dissolve in water
Ex. Phospholipids, hydrophobic ends face the inward and hydrophilic face outward to aequous environment
Macromolecules:
Chains of chemical units linked end-to-end → key to all cellular processes
Ex. 70% of bacteria cell is water.
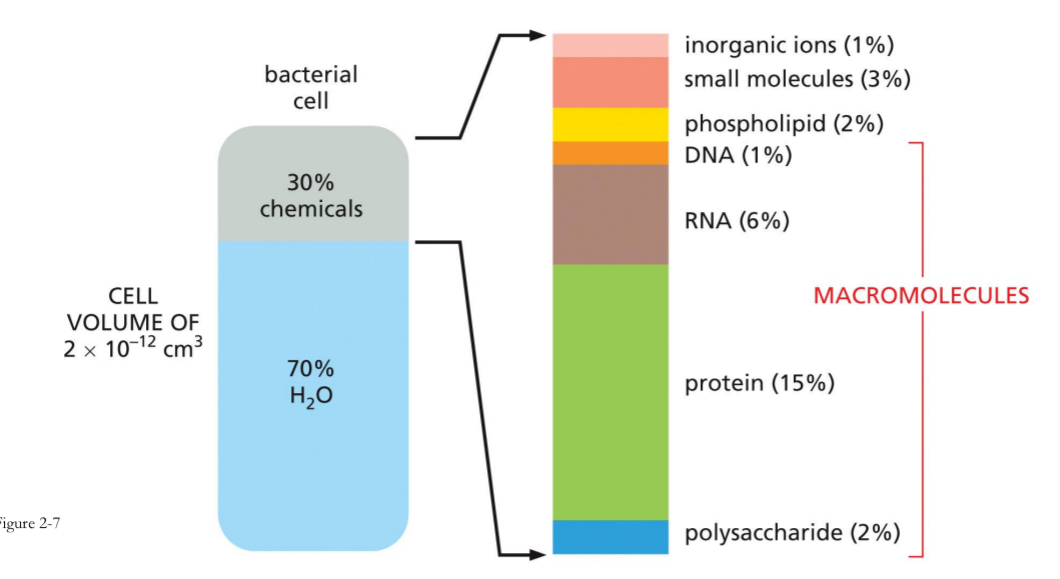
Macromolecules include
DNA
RNA
Protein
Polysaccharides
Monomers Make Up Macromolecules
Monomers of macromolecules are joined together by covalent bonds.
Macromolecules are polymers of building blocks known as monomers
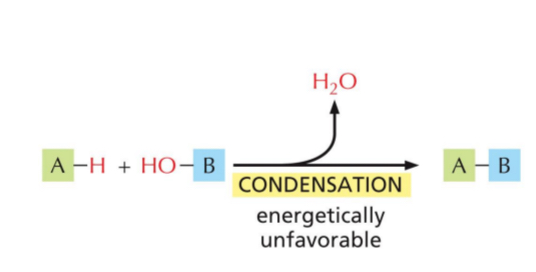
Polymers form by joining monomers → condensation (water is removed)
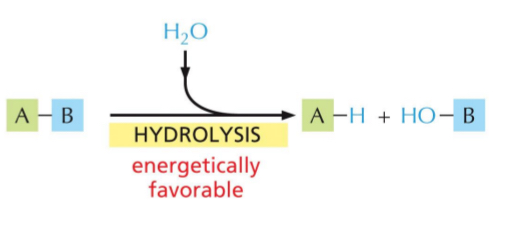
Polymers are broken down into monomers → hydrolysis (water is added)
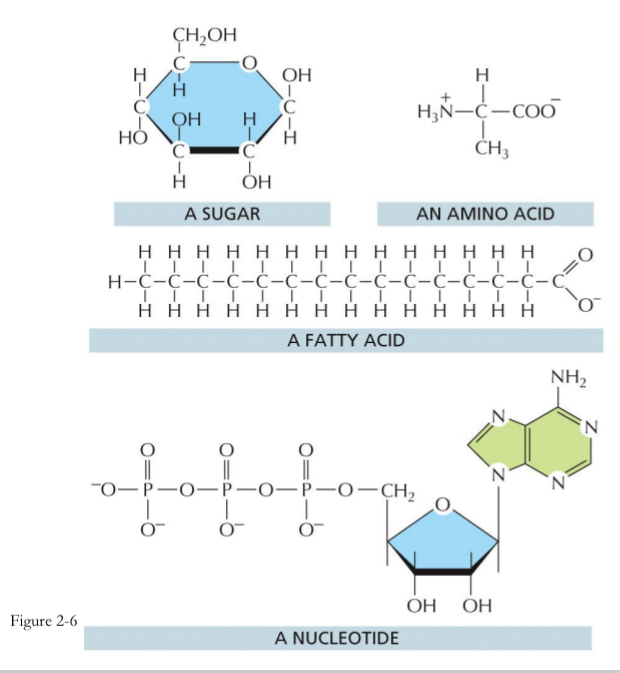
Sugars form
polysaccharides, glycogen, and starch (In plants)
Fatty acids form
fats and membrane lipids
Amino acids form
proteins
Nucleotides form
nucleic acids
Condensation
Removes water
Energetically unfavourable
Hydrolysis
Add water
Energetically favourable reaction
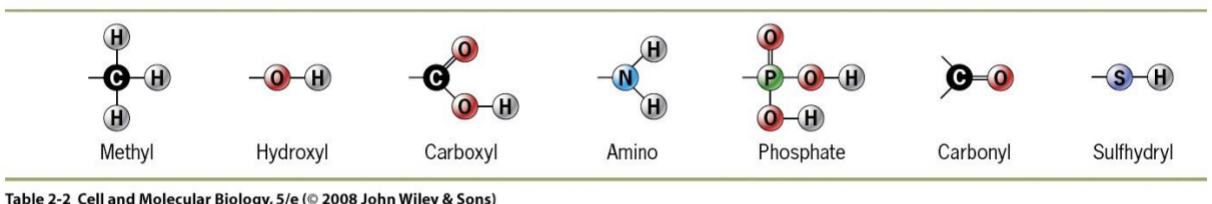
Functional Groups
particular atom groupings that behave as a unit.
These affect the properties of biomolecules (e.g. change chemical reactivity) because:
Contain electronegative atoms (N,O,P,S)
Can make molecules more polar or more reactive
May confer a positive or negative charge due to ionization
Memorize functional groups
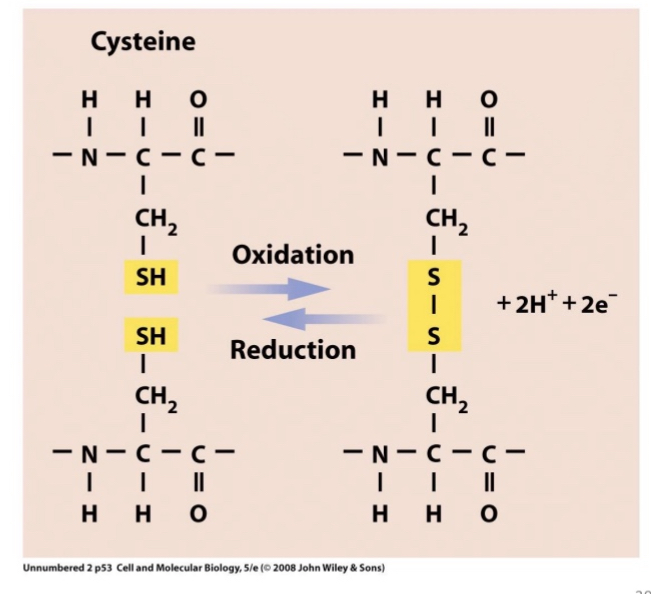
Ex. Sulfhydral present in certain amino acids that form bonds with other proteins that also have sulfer = forming a disulphide bridge → important in joining subunits of protein
Biomolecules characteristics
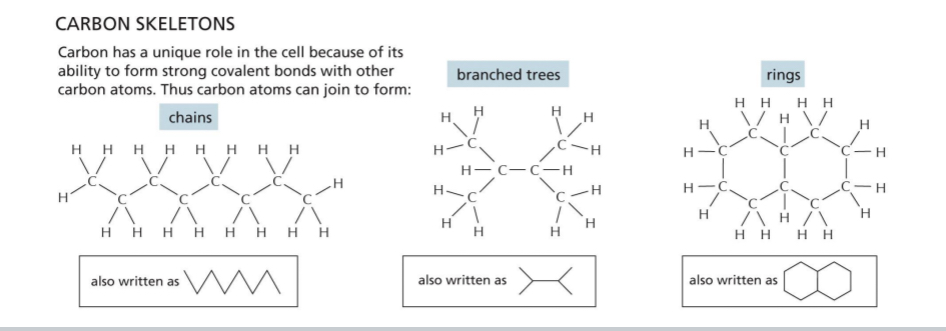
Biomolecules centred around Carbon → carbon binds up to 4 other atoms
Long chains of carbon atoms used to construct biological molecules
Linear, branched or cyclic
Simplest group of biological molecules → hydrocarbons (C-H)
Certain hydrogens is often replaced by “functional groups”
Carbohydrates
General formula: (CH2O)n
Important sugars in cell metabolism have 3-7 carbons
3 sugars = trioses; 4 sugars = tetroses; 5 sugars = pentoses; 6 sugars = hexoses
Carbonyl internal position – forms ketone = ketose
Carbonyl at one end – forms aldehyde = aldose
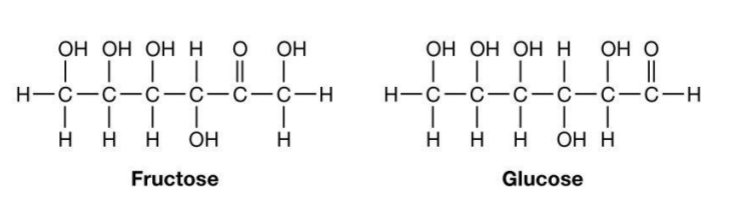
Carbonyl internal position – forms
ketone = ketose
Carbonyl at one end – forms
aldehyde = aldose
Monosaccharides – Closed Ring Structures
Sugars with 5 or more carbons form a closed ring structure.
Forms ring formation
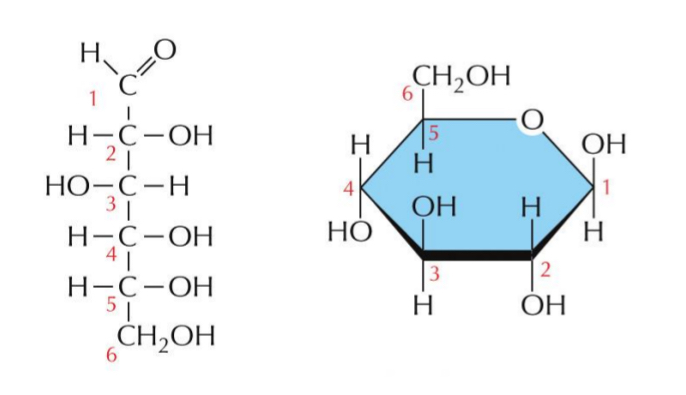
Ring formation
in aqueous solution, the aldehyde or ketone group of a sugar molecule tends to react with a hydroxyl group of the same molecule, closing the molecule into a ring
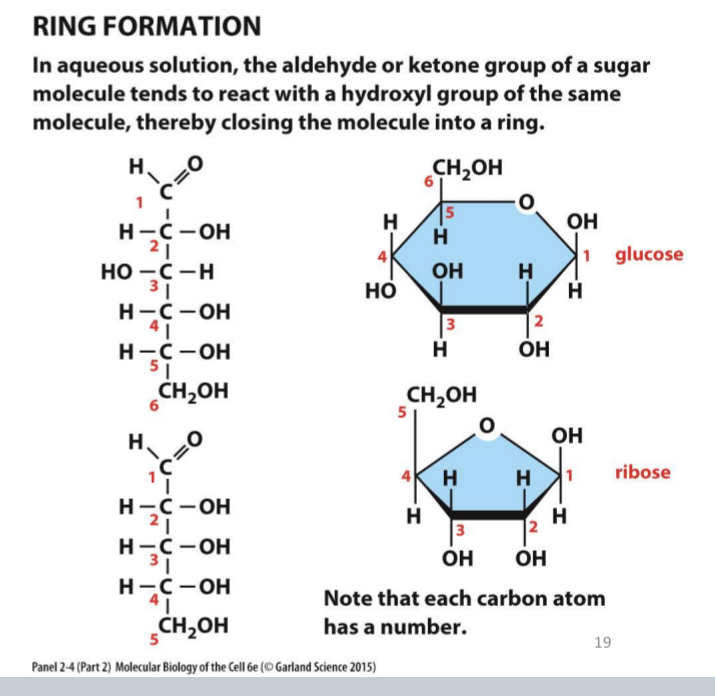
Example: Glucose
Glucose = 6-carbon ring
OH of C1 projects below the plane of the ring - α-glucose
OH of C1 projects up from the plane of the ring - β-glucose
As soon as one sugar is linked to another the alpha and beta form is frozen
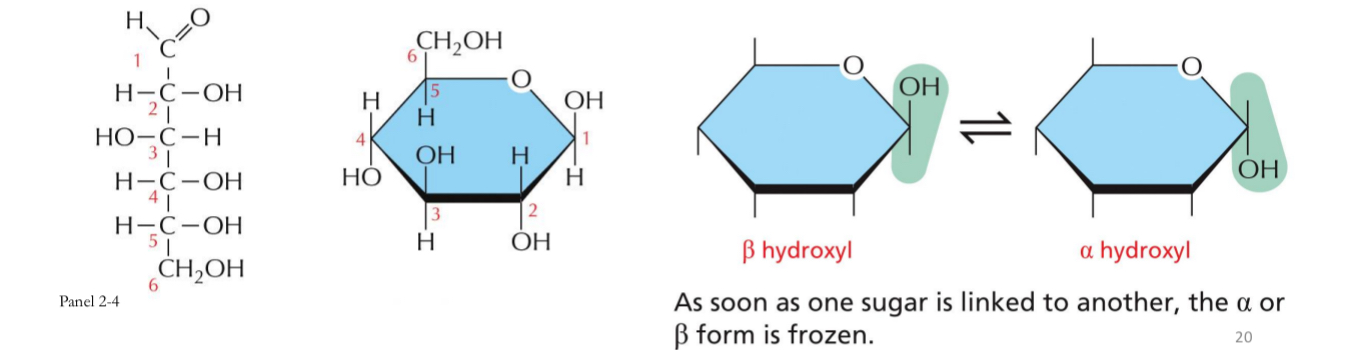
α-glucose
OH of C1 projects below the plane of the ring
β-glucose
OH of C1 projects up from the plane of the ring - β-glucose
Isomers
Same formula but different arrangement
ex. Glucose, galactose and mannose have the same formula (C6H12O6) but differ in arrangement of groups around one or two atoms
The small differences make only minor changes in the chemical properties of the sugar. But they are recognized by enzymes and other proteins and therefore can have major biological effects
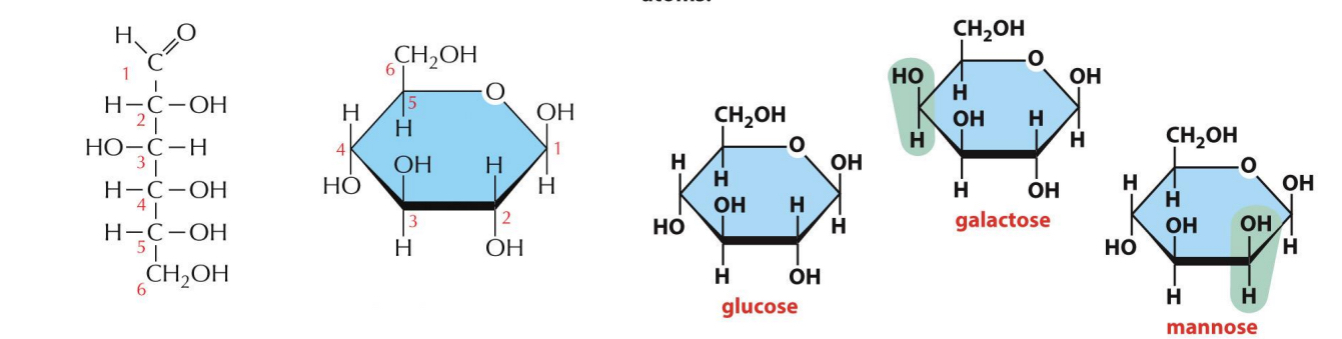
Carbohydrate Polymers
Covalent bond formed between C1 of one sugar and hydroxyl (OH) of another sugar
Generates C-O-C linkage between sugars
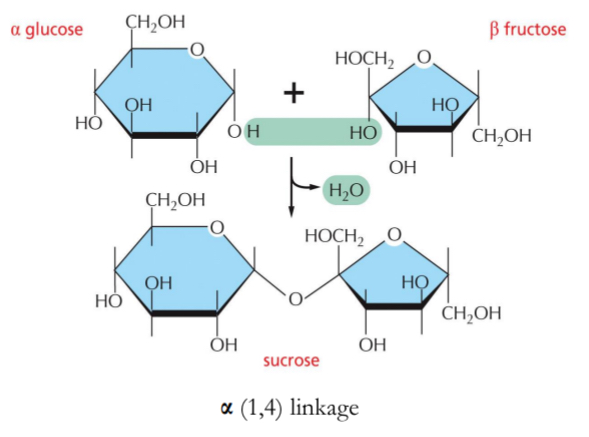
Disaccharides:
Two monosaccharides covalently bonded together → energy storage, e.g., sucrose, maltose, lactose
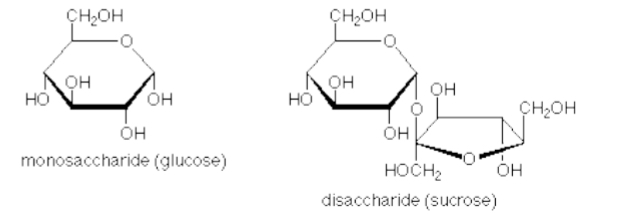
Oligosaccharides:
a small chain of sugars → when attached to lipids or proteins = glycolipids or glycoproteins
Polysaccharides:
a long chain of sugars
very large molecules with a structural or storage function
E.g.: chitin (structural) , cellulose, starch, glycogen (in liver, and we can survive off just glycogen for 48 hrs. )
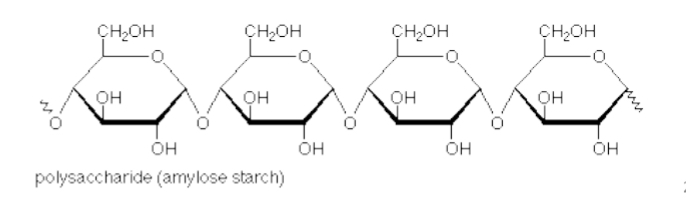
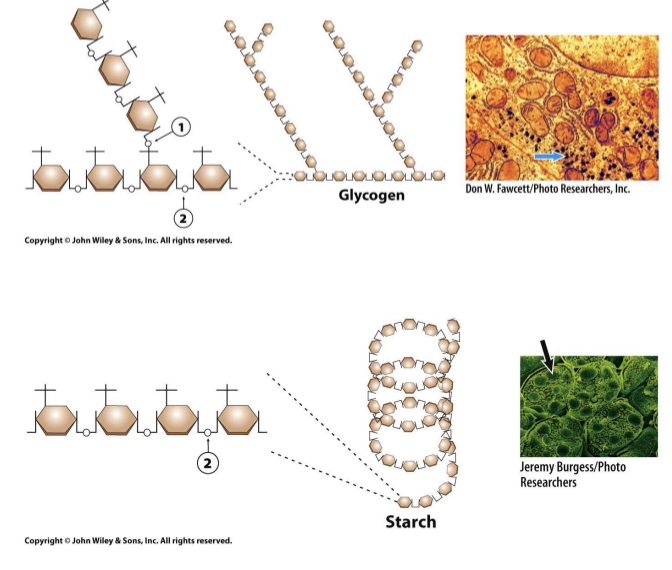
Glycogen and Starch contain
α(1,4) linkages resulting in long, branched chains (glycogen) or coils (starch).
Glycogen or starch granules (where the arrow is pointing) can be broken down to get glucose
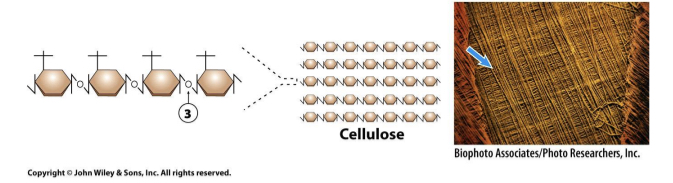
Cellulose contains
β(1,4) linkages resulting in long branches.
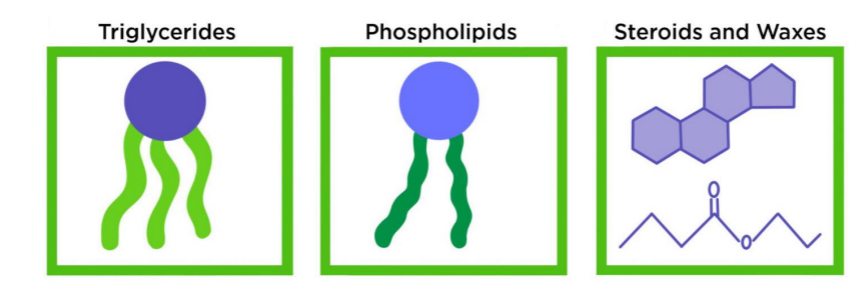
Lipids
A large group of nonpolar biological molecules → dissolve in organic solvents but not in water (lack polar groups = hydrophobic)
Composed mainly of C,H and O
Lipids with important cell functions: fats, phospholipids, steroids
3 types of lipids
Triglycerides
Phospholipids
Steroids and waxes
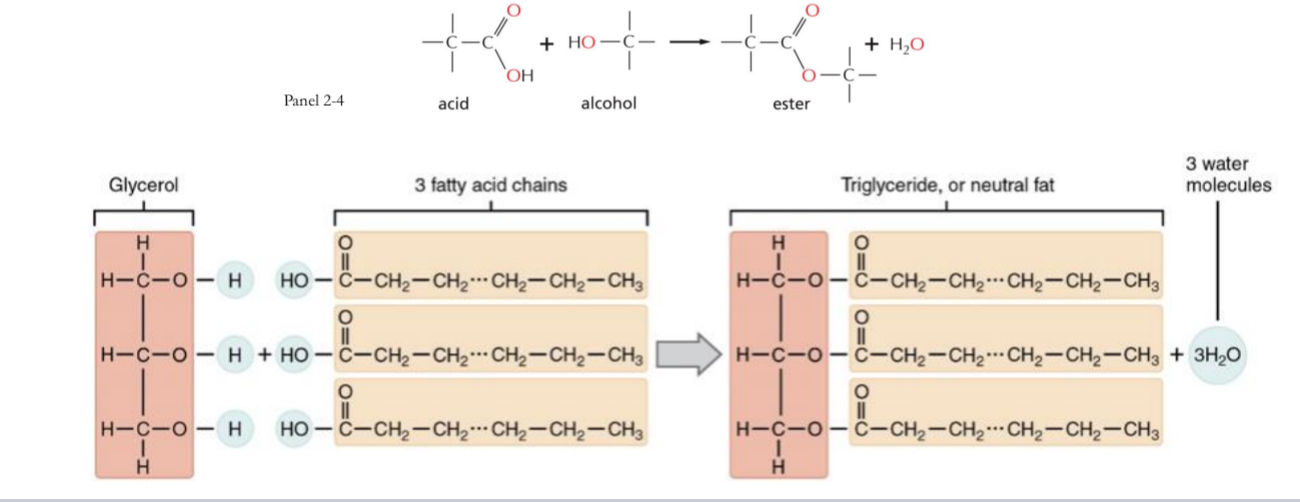
Fats = triacylglycerol
= glycerol + 3 fatty acids
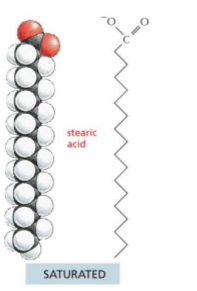
Lipids – Fatty Acids
long hydrocarbon chains with a single carboxyl (COOH) at one end
Vary in length
Fatty acid with No double bonds
saturated
Can be solid
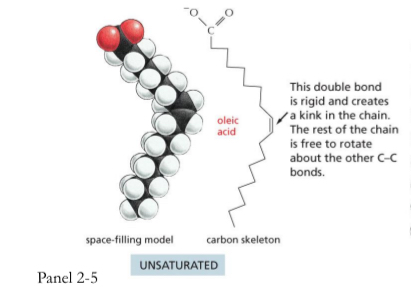
Fatty acid with Double bonds
unsaturated
This double bond is rigid and creates a kink and bending in the chain. The rest of the chain is free to rotate about the the other C-C bonds
They can’t pack as tightly.
Important in plasma membrane where things need to move around
Liquid state
Triacylglycerols
Form large spherical fat droplets in the cell cytoplasm
Molecules don’t have a change in an aqueous environment → cluster together
What links fatty acids to glycerol to form triacylglycerol
Ester bonding
Esters
Are formed by combining an acid and an alcohol with byproduct of alcohol
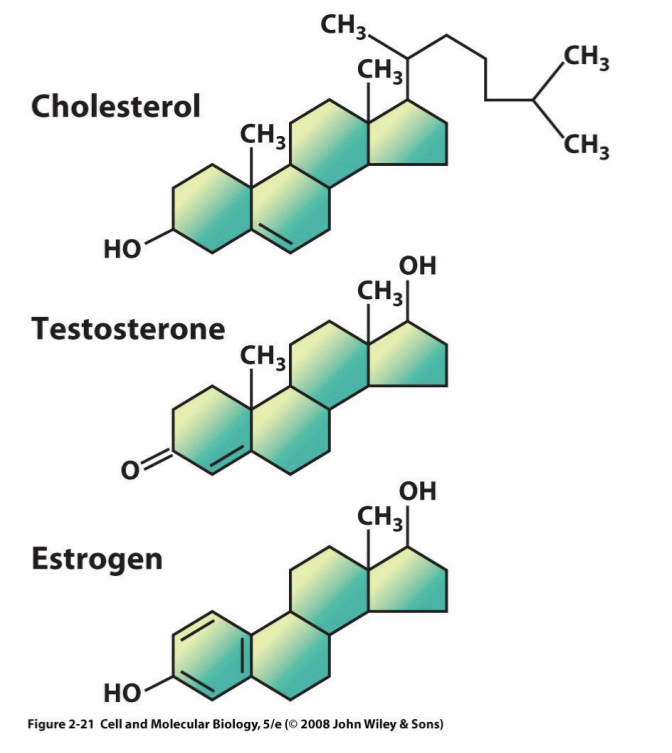
Lipids – Steroids
Complex ring structures → 4 hydrocarbon rings
E.g.: Cholesterol = important animal plasma membrane component
Building blocks of many steroid hormones
Not present in plant cells → cholesterol- free
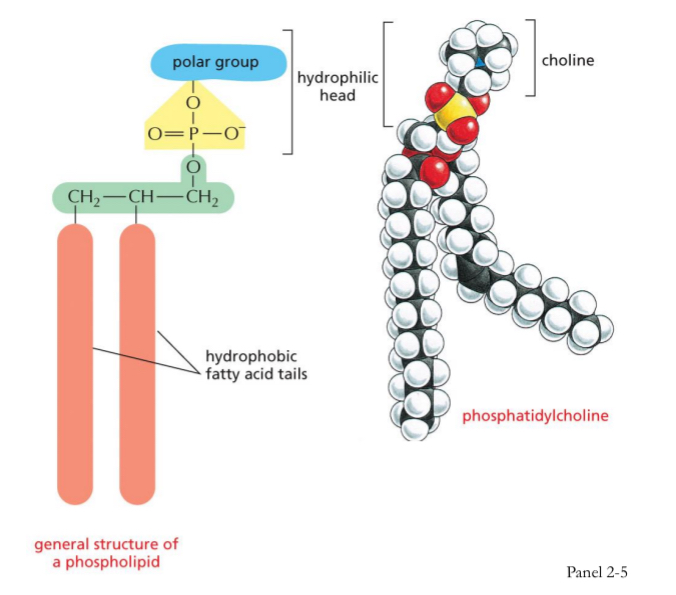
Lipids – Phospholipids
Composed of glycerol + 2 fatty acids + phosphate group
Major component of plasma and organelle membranes
Hydrophilic on one end and hydrophobic on the other → amphipathic (polar head group and non polar tail)
Positively charged choline group attached to the phosphate = phosphatidyl choline
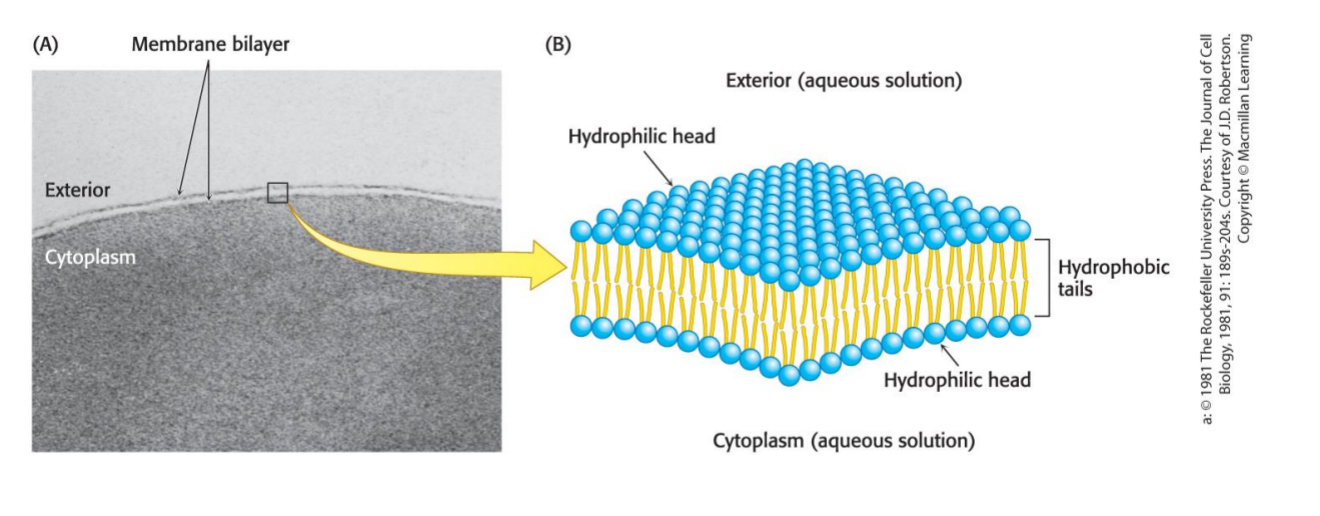
Lipid bilayers are formed by
phospholipids
DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are
polymers of nucleotides
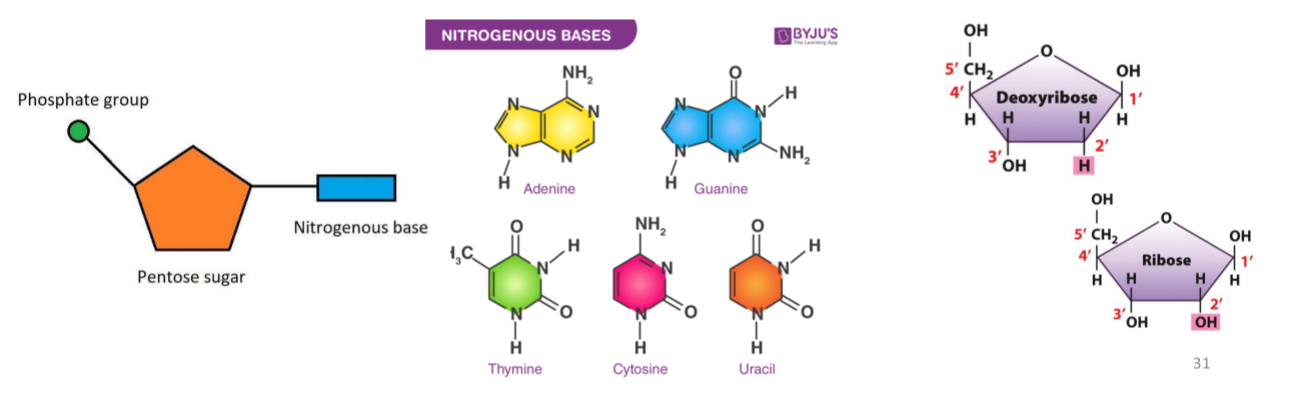
Nucleotides
5-carbon sugar + nitrogenous base + phosphate
Nucleosides
5-carbon sugar + nitrogenous base (no phosphate)
What type of sugar does DNA have
deoxyribose sugar
What type of sugar does RNA have
ribose sugar
Purines
adenine (A) and guanine (G)
Double ring structure
Pyrimidines
cytosine (C) and thymine (T) in DNA
T is replaced by Uracil in RNA
Single ring
Nucleotides And phosphodester bond
Nucleotides are joined by sugar-phosphate linkages
3’ hydroxyl attached to 5’phosphate of the adjoining nucleotide → phosphodiester bond
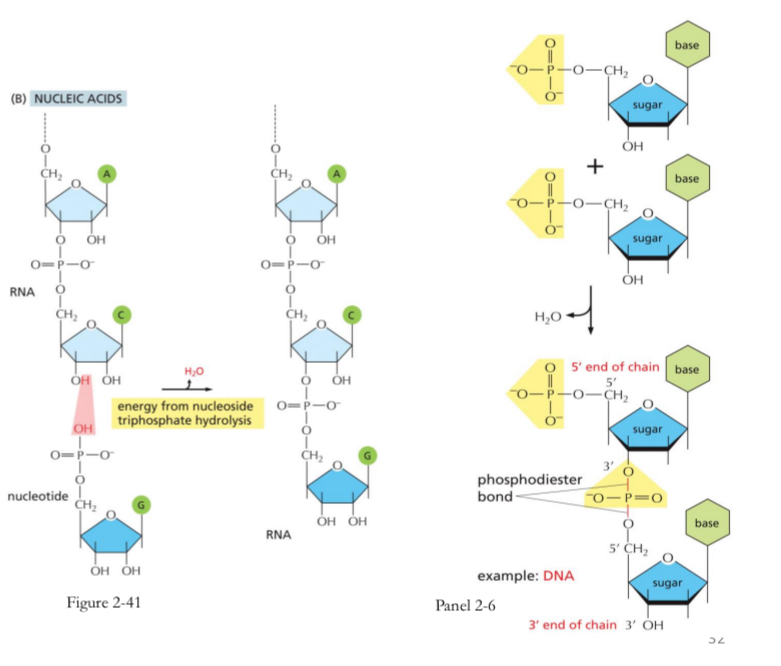
Base pairing → hydrogen bond
DNA double helix formed by base pairing of a purine with a pyrimidine
A bonds with T = 2 hydrogen bonds
G bonds with C = 3 hydrogen bonds
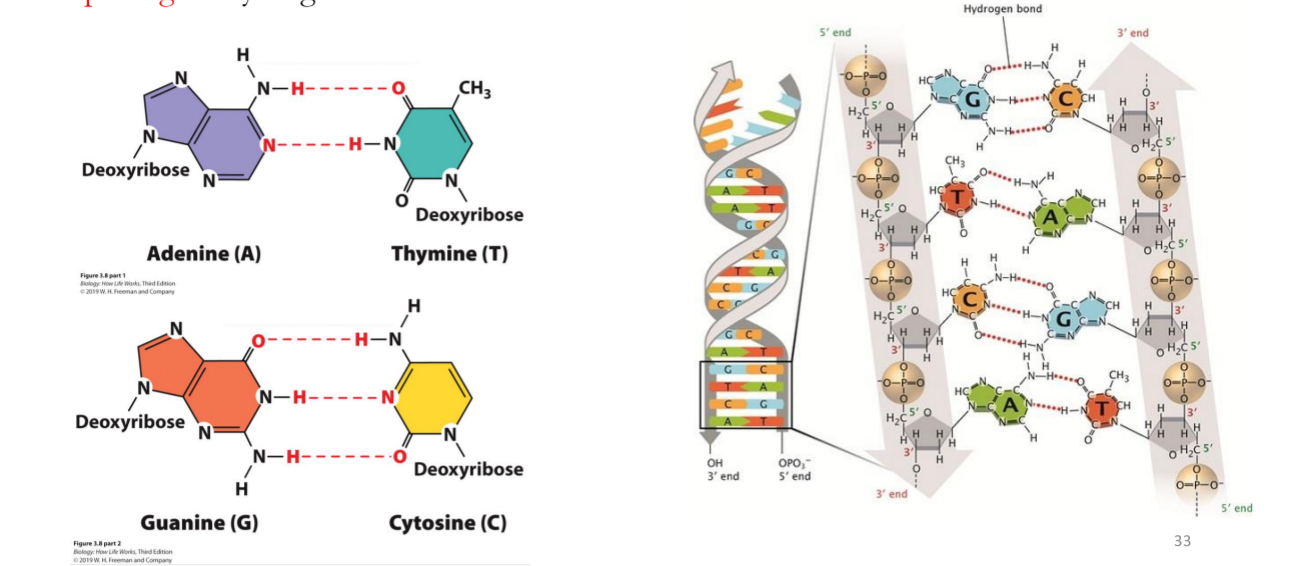
A bonds with
T = 2 hydrogen bonds
Bond will break faster and easier than G-C bonds because of one less hydrogen bonds
G bonds with
C = 3 hydrogen bonds
Nucleotides serve multiple functions
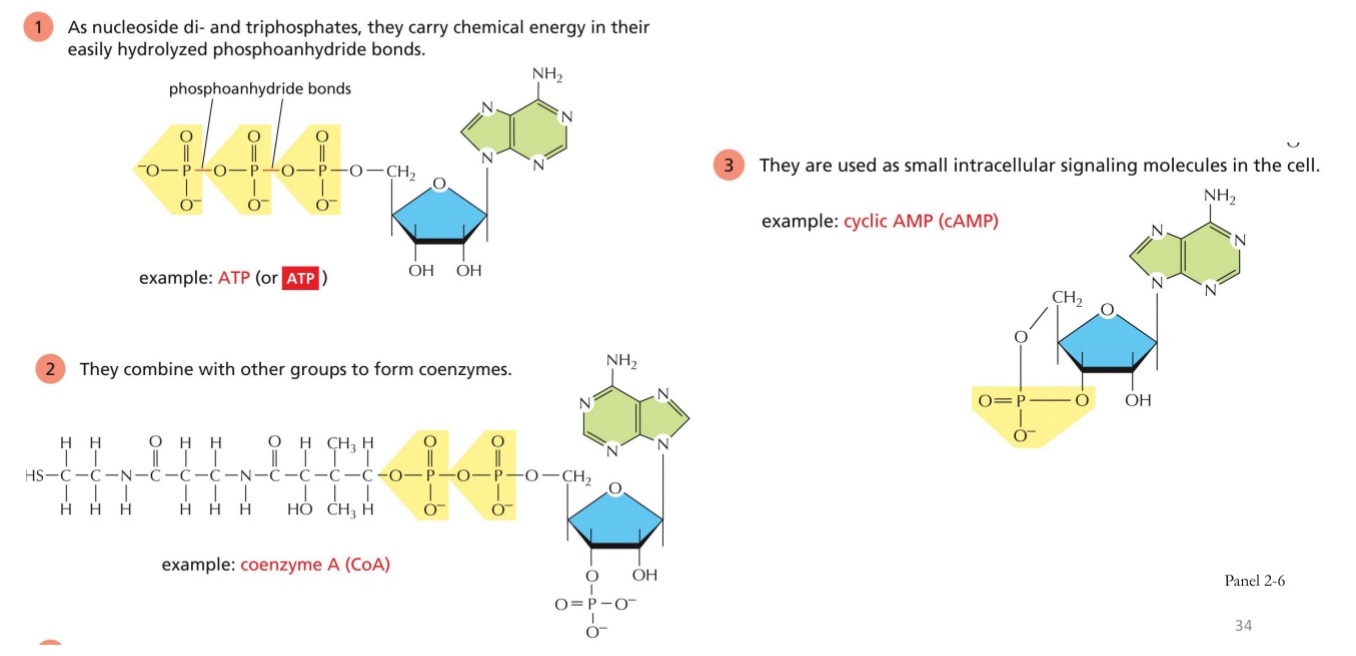
Proteins
About ~ 1 x 104 proteins made in every mammalian cell per second!
Carry out almost all cellular functions. For example:
Enzymes - accelerate chemical reactions in the cell
Signaling - kinases, phosphatases are involved (initiate reactions that lead to another reaction)
Hormones - long range messenger molecules
Growth factors
Membrane receptors - communication between cells
Cell movement - cytoskeleton
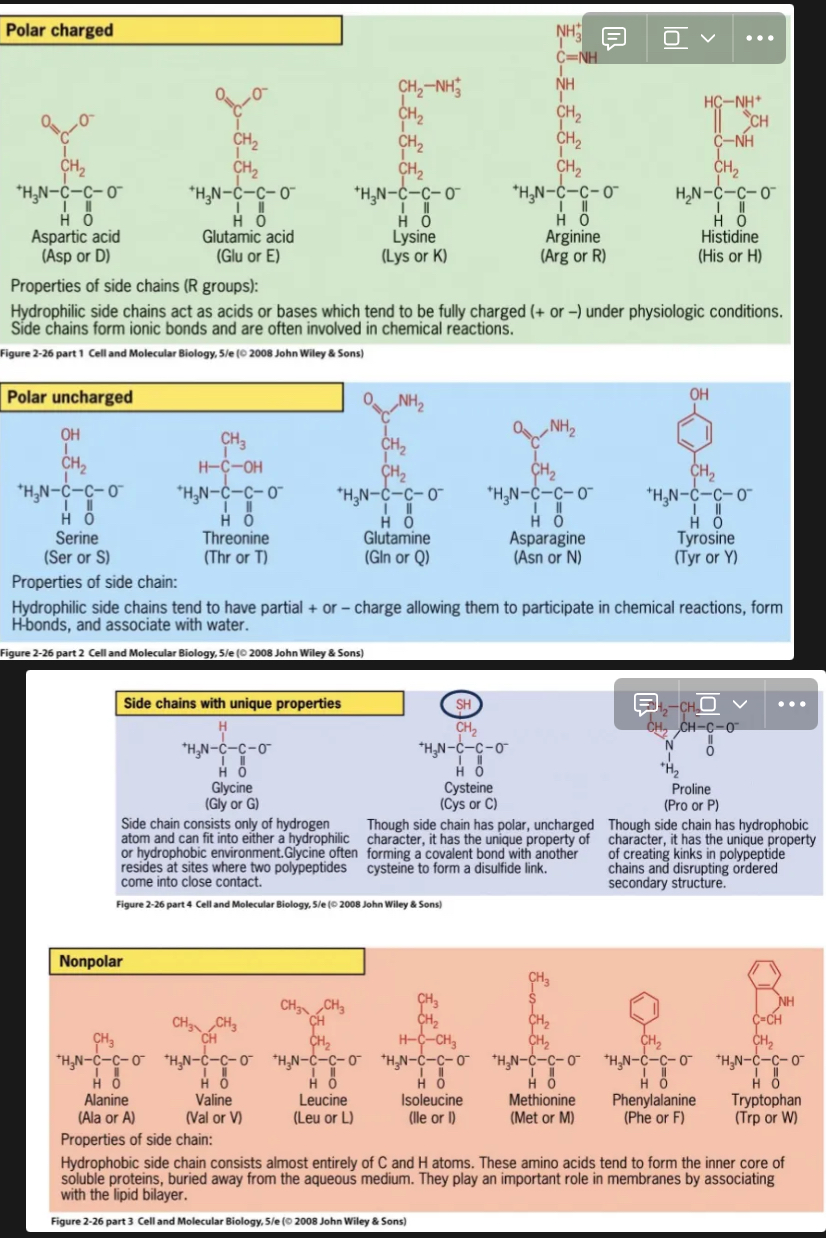
Amino Acids
Composed of H, C, O, N and also S or P
20 different types
Amino (NH2) and Carboxyl (COOH) groups
These groups are separated by a single carbon (α-carbon)
R groups (side chains) give amino acids their variability
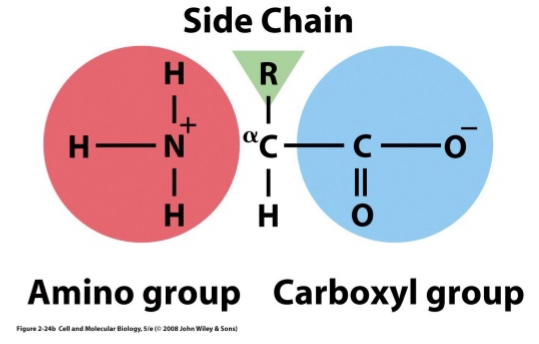
R - Groups
Four categories of R groups:
Polar charged: form ionic bonds
Polar uncharged: form H bonds
Nonpolar
Other e.g. sulfhydryl or cysteine
MEMORIZE THE 14 AMINO ACIDS codes
How does cell know which order to put amino acids in
mRNA
What dictates the sequence of mRNA
DNA
Cysteine amino acids can
form disulfide bonds in oxidizing conditions
Peptide Bonds
Carboxyl group of one amino acid becomes attached to the amino group of another
Join amino acids
Forms polypeptide chains → proteins
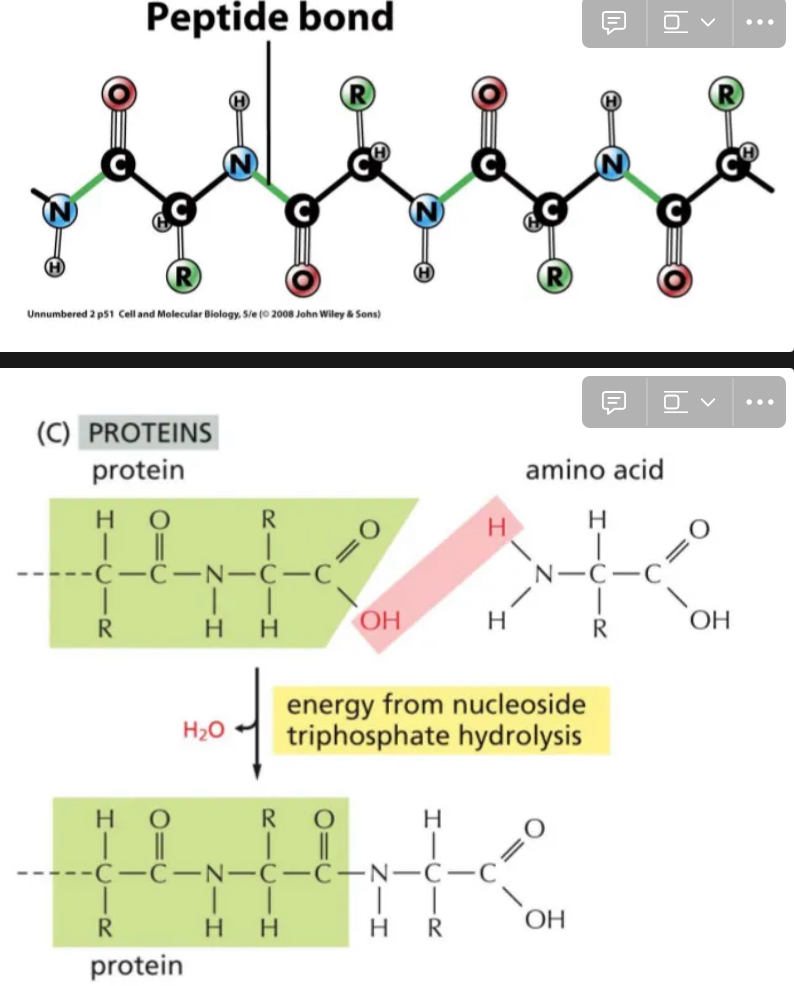
What forms peptide bonds in cells
Ribosomes use energy to form this bond, then do elongation
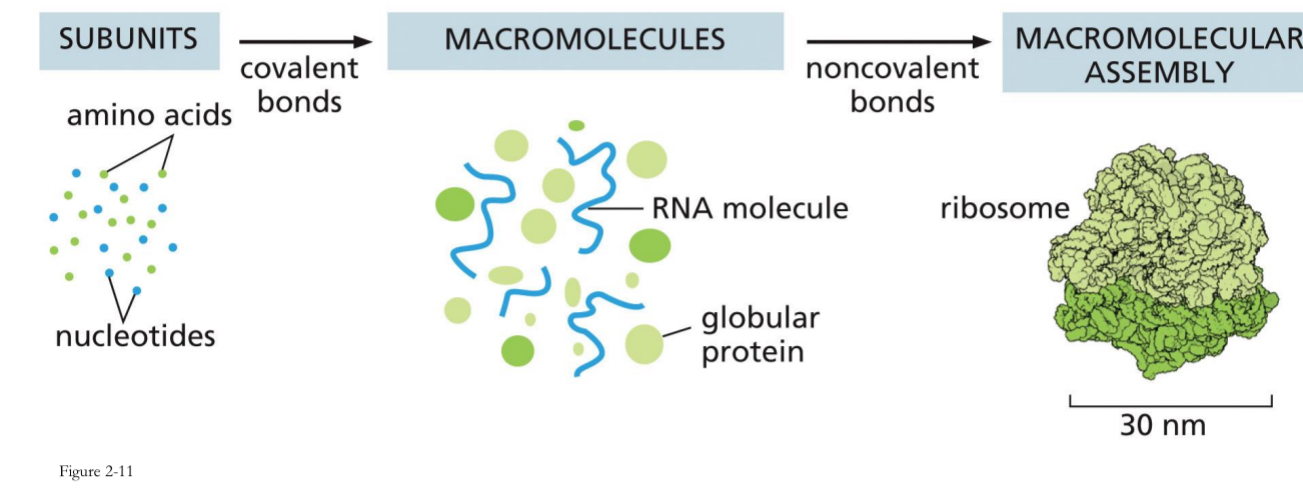
Macromolecules can assemble in complexes
Important reason for complexes is when everything is in close proximity is in the cell.
By bringing all the necessary components of a biological process together in a single, coordinated protein complex, the cell can dramatically increase the speed, efficiency, and specificity of its functions.
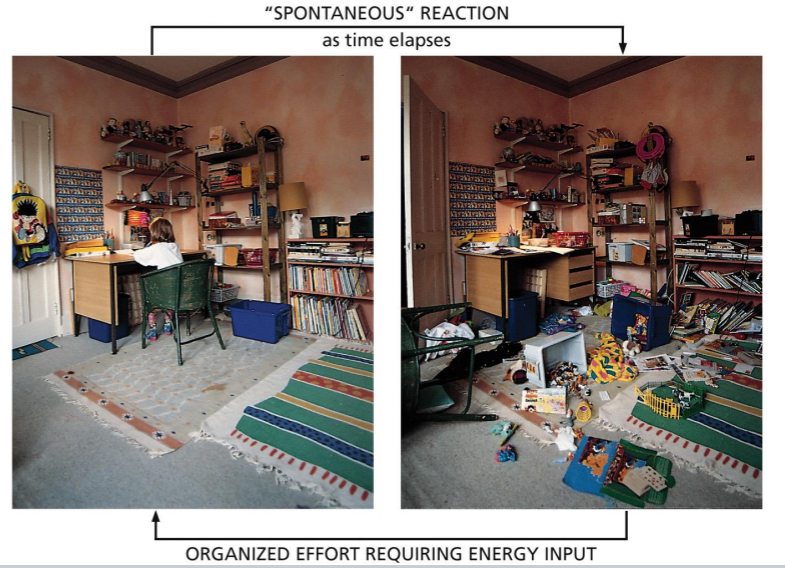
Macromolecule Synthesis and Breakdown
reversing disorder is not spontaneous
total entropy of an isolated system can only increase over time; it can never spontaneously decrease.
Second law of thermodynamics → cannot reverse the state of a system without increasing entropy of surrounding
Need release of heat (energy conversion)
Metabolism
Anabolic and catabolic pathways
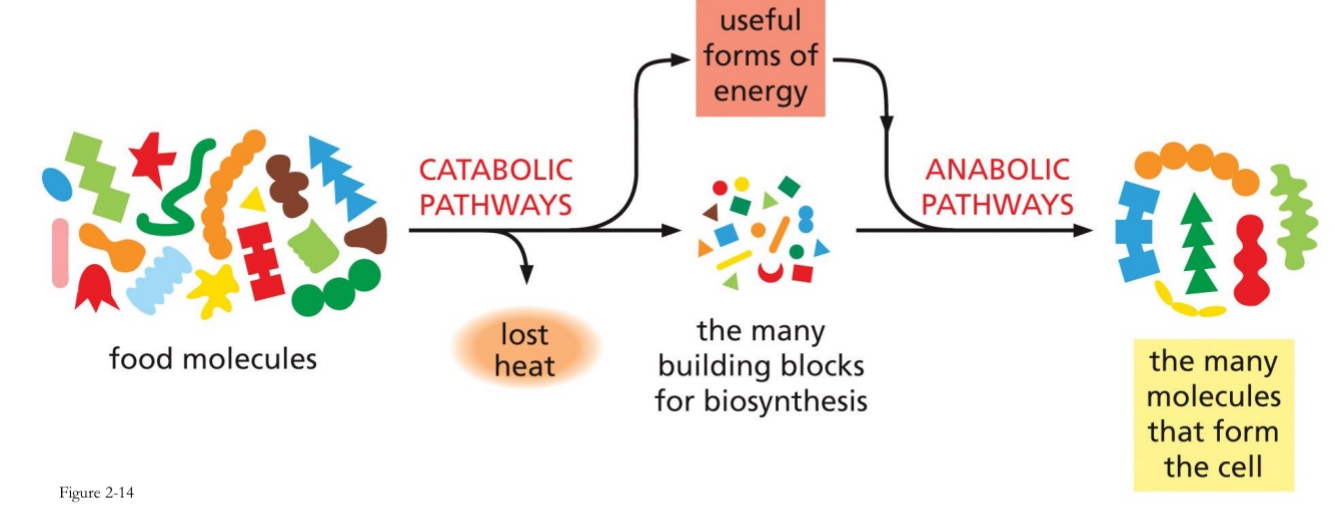
Anabolism
the synthesis of complex molecules in living organism from simpler ones together with the storage of energy
Catabolism
Break down larger molecules to smaller usable units for biosynthesis
Produce useful forms of energy
Use energy in anabolic pathways to make other molecules
Catalysts and Cell Metabolism
Cell metabolism pathways comprise many enzymes.
Ex glycolysis has 7 main steps
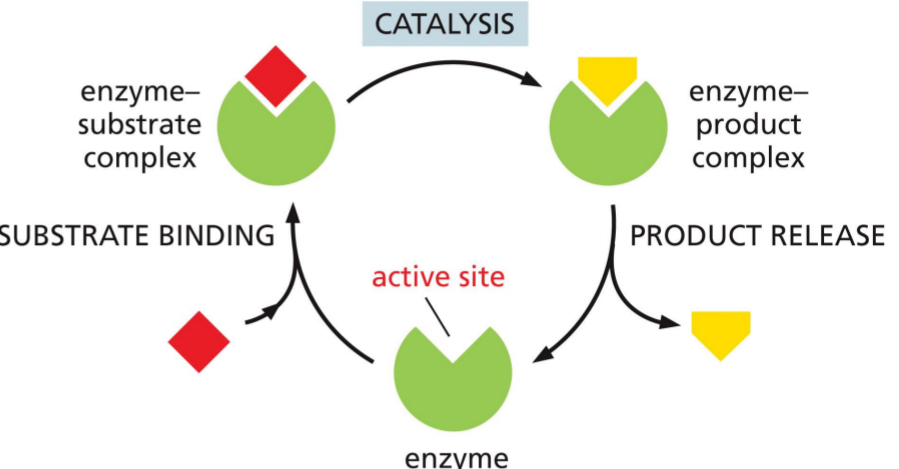
How Enzymes Work
Enzymes (catalysts) do not change during reaction.
Enzymes bring down the energy barrier
Enzyme (it’s a protein) has an active site. The active site is a specific organization of amino acid into a 3D shape, such that substrate can fit into the pocket
The substrate fits very tightly and formed enzyme substrate complex
Carry out catalysis
Now have enzyme product complex.
Product get released and enzyme has an empty active site again
Enzyme doesn’t get used just recycled
This cycle continues until protein gets degraded
Enzymes Can Drive Specific Pathways
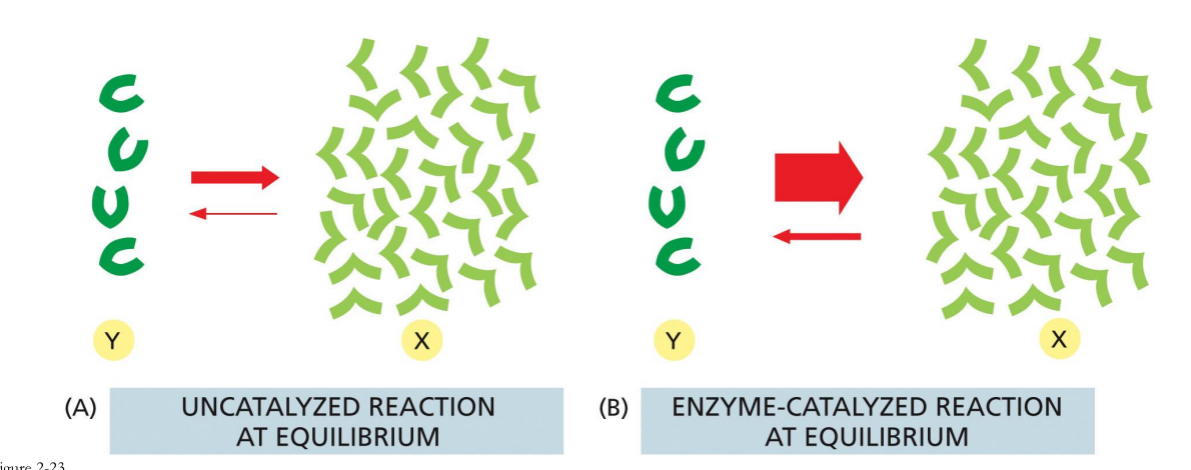
Enzyme catalyzed reactions can reach equilibrium much faster.
Uncatalyzed reaction at equilibrium. The units (Y) will form X
Not a whole lot is made. There are also unfavourable reverse reactions occurring
Enzyme catalyzed reaction at equilibrium: drives the reaction in one direction. No more reverse reactions
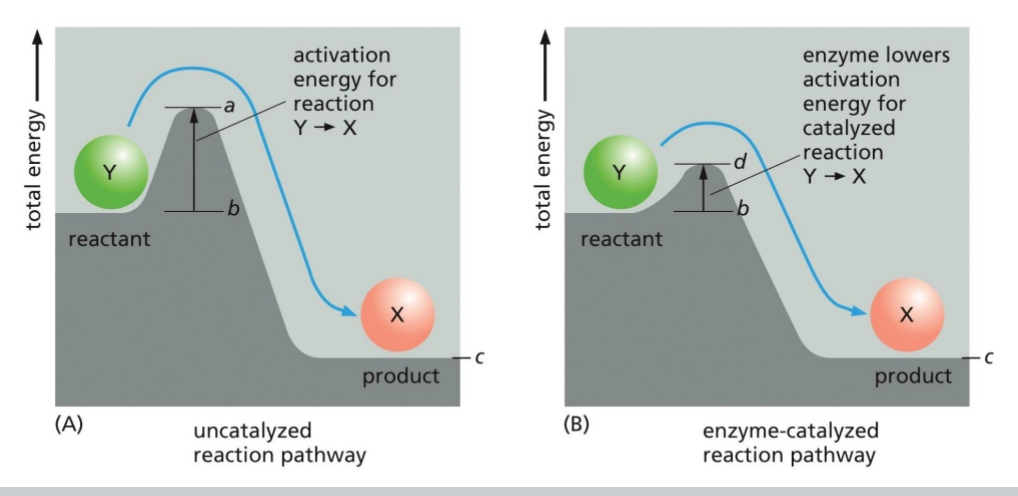
Enzymes Reduce Activation Energy Barriers
Reduced activation energy barriers → increased probability of reaction.
Allows for chemical reactions to proceed at normal temperatures
Lowered energy activation means this reaction will occur easier, and faster
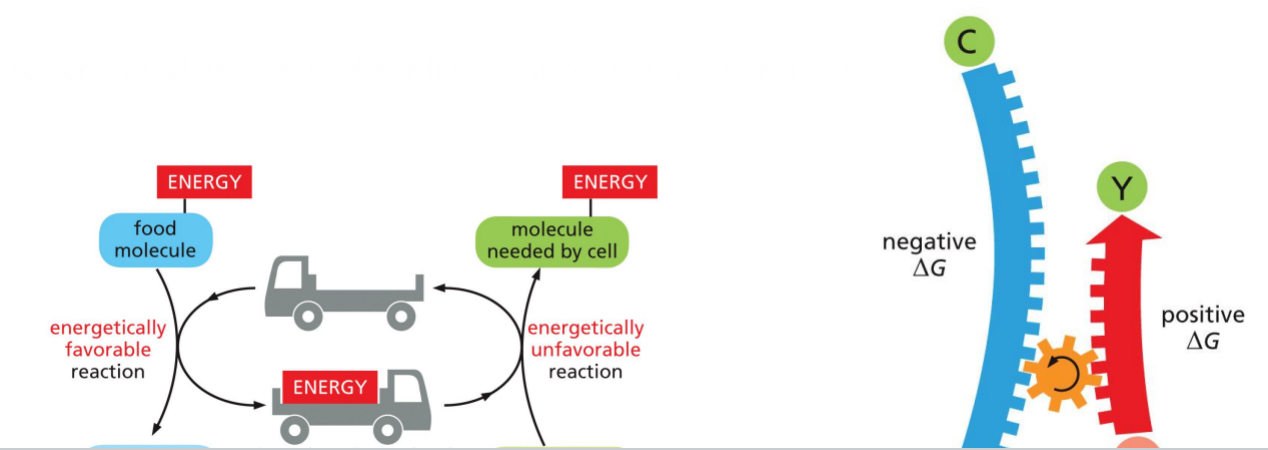
Enzymes Cannot Force Energetically Unfavourable Reactions
∆G (Gibbs free energy) is a measure of the change in the amount of energy available to do work.
Favourable reactions decrease the ∆G (negative ∆G), release energy and increase the disorder of the surroundings. → occurs spontaneously
Unfavourable reactions increase the ∆G (Positive ∆G), require energy and decrease the disorder of the surroundings → only occurs if it is coupled to a second energetically favourable reactions
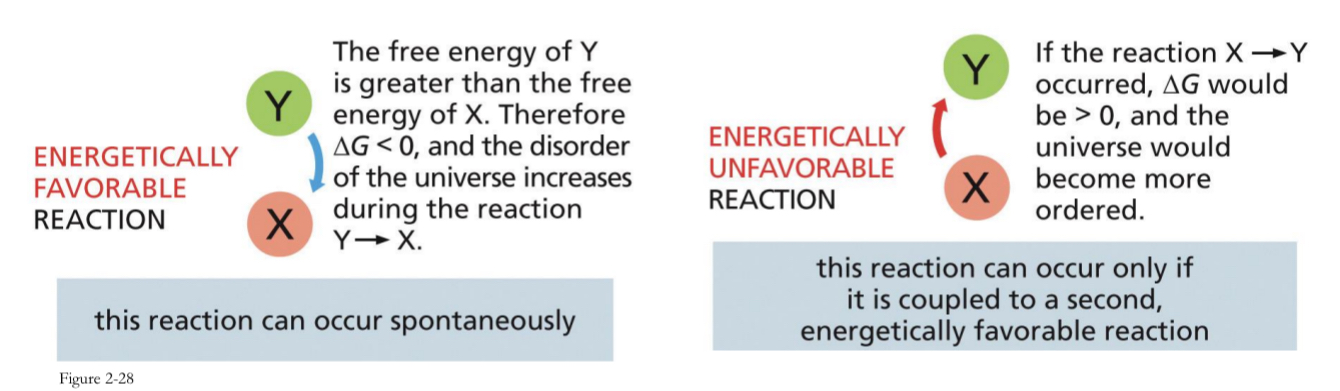
Favourable reactions
decrease the ∆G (negative ∆G), release energy and increase the disorder of the surroundings. → occurs spontaneously
Unfavourable reactions
increase the ∆G (Positive ∆G), require energy and decrease the disorder of the surroundings → only occurs if it is coupled to a second energetically favourable reactions
This will not happen naturally or spontaneously
Cells Utilize Reaction Coupling
Use an energetically favourable reaction to drive an energetically unfavourable reaction
Free energy change < 0 (disorder of surroundings increases)
Energy carrier is the truck below