Lecture 19 - BIoenergetics
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
What is the reaction for the complete oxidation of glucose?
C₆H₁₂O₆ + 6 O₂ → 6 CO₂ + 6 H₂O + energy
What is the standard free energy change (ΔG°′) for the oxidation of glucose?
ΔG°′ = –2,840 kJ/mol glucose
Why is glucose oxidation important in cells?
It provides a major source of free energy for cellular activities—one of the most important metabolic processes in living cells.
How do cells conserve energy released from glucose oxidation?
They break down glucose through many enzyme-catalyzed steps (a pathway), mostly involving small free energy changes.
When is energy captured during glucose catabolism, and in what form?
Some steps have large negative ΔG°′, allowing energy to be trapped in small usable packets (“quanta”) to drive other reactions.
What are two major forms of biological energy quanta?
Reduced electron carriers (e.g. NADH, NADPH, FADH₂, FMNH₂)
Nucleoside triphosphates (e.g. ATP, GTP)
How are biological energy quanta used in cells?
They are shared with other molecules and pathways to provide energy for cellular work.
Why are enzymes essential in bioenergetics?
Even reactions with negative ΔG° (spontaneous) may not occur without enzymes, which provide a reaction mechanism.
What type of reaction is the primary source of biological energy?
Oxidation-reduction (redox) reactions—transfers of electrons.
Why are redox reactions important in biology?
Because carbon atoms in biological molecules can exist in multiple oxidation states, allowing energy to be released during redox reactions.
How does the oxidation number of a carbon atom change when it loses hydrogen atoms (protons)?
When carbon loses hydrogen atoms (which are less electronegative than carbon), its oxidation number increases. This is because the carbon is losing electron density, meaning it becomes more oxidized.
What does a more negative oxidation state of carbon indicate?
A more negative oxidation state means the carbon is more reduced—it has more electron density, usually due to bonds with hydrogen (which is less electronegative than carbon).
What is the most oxidized form of carbon commonly found in living systems?
Carbon dioxide (CO₂) is the most oxidized form of carbon in living organisms. The carbon in CO₂ has an oxidation state of +4.
In a redox reaction, what happens to the electron donor and electron acceptor?
The electron donor (initially reduced) loses electrons and becomes oxidized; the electron acceptor (initially oxidized) gains electrons and becomes reduced.
Why can’t a redox reaction occur between A_ox + B_ox or A_red + B_red?
Because A_ox has no electrons to donate, and A_red has nowhere to transfer electrons to if B is also reduced.
How is the direction of electron flow determined in a redox reaction between two molecules?
Electrons flow from the molecule with lower electron affinity to the molecule with higher electron affinity.
What is the standard reduction potential (E°), and how is it measured?
E° is a measure of a molecule’s affinity for electrons, determined relative to the proton reduction half-reaction (H⁺ + e⁻ → ½ H₂) at standard biochemical conditions (1 atm, 298 K, 1 M). It is measured by combining species and observing electron flow direction; in units of volts (V)
How is the standard reduction potential measured experimentally?
Using a voltmeter to measure the voltage difference between two half-cells (in volts or millivolts), each representing a redox half-reaction. The voltage reflects the electron affinity difference.
What is the Standard Hydrogen Electrode (SHE)?
It is the reference electrode used for measuring reduction potentials, where the half-reaction is H₂ gas evolving or consuming protons (H⁺ + e⁻ ⇌ ½ H₂), defined as 0 V by convention.
What happens in the oxidation of Fe²⁺?
Fe²⁺ loses an electron and becomes Fe³⁺ : Fe²⁺ → Fe³⁺ + e⁻ (oxidation).
What happens in the reduction of Fe³⁺?
Fe³⁺ gains an electron and becomes Fe²⁺ : Fe³⁺ + e⁻ → Fe²⁺ (reduction).
What is the standard reduction potential (Eº) of the Fe³⁺/Fe²⁺ redox pair?
+0.77 Volts (V), meaning Fe³⁺ has a high affinity for electrons and tends to accept electrons from species like H₂.
What is the half reaction for the reduction of protons (H⁺) under standard conditions?
H⁺ + e⁻ → ½ H₂(g), which is used as the reference electrode (standard hydrogen electrode, SHE) with Eº = 0 V.
Describe the overall reaction when Fe³⁺ and H₂ are combined under standard conditions.
½ H₂ + Fe³⁺ → Fe²⁺ + H⁺, with Fe³⁺ acting as the electron acceptor. (look at table)
What is the redox behavior of the NADH/NAD⁺ pair?
NADH donates electrons:
NADH → NAD⁺ + H⁺ + 2 e⁻ (oxidation).
NAD⁺ gains electrons:
2 H⁺ + 2 e⁻ → H₂ (reduction).
What is the standard reduction potential (Eº) for the NADH/NAD⁺ couple?
Eº = –0.32 V, indicating NADH has a lower affinity for electrons than H⁺ and thus donates electrons to protons.
How does electron flow occur between NADH and the hydrogen electrode under standard conditions?
Electrons flow from NADH to the hydrogen electrode, demonstrating that NADH is oxidized and H⁺ is reduced: NADH + H⁺ → NAD⁺ + H₂.
How does standard reduction potential (E°′) help predict the direction of electron flow in a redox pair?
Electrons flow from the molecule with the more negative E°′ (stronger reducing agent) to the one with the more positive E°′ (stronger oxidizing agent). A positive ΔE°′ (E°′ acceptor – E°′ donor) means the reaction is spontaneous.
What is the significance of a positive ΔE°′ in redox reactions?
A positive ΔE°′ indicates that the reaction is favoured and corresponds to a negative ΔG, meaning the reaction can do work.
What is the formula that relates standard free energy change (ΔG°′) to redox potential (ΔE°′)?
ΔG°′ = –nℱΔE°′, where n is the number of electrons transferred, ℱ is Faraday’s constant, and ΔE°′ is the standard reduction potential.
What is Faraday’s constant and what are its units?
Faraday’s constant (ℱ) is 96.1 kJ/(volt·mol) and represents the amount of energy per mole of electrons per volt of potential difference.
How can oxidation of carbon atoms generate useful energy?
Oxidation of carbon atoms causes electron flow; if this flow is coupled to other reactions, the released energy can be harnessed to do work.
Why must cells capture energy in small steps during glucose oxidation?
The oxidation of glucose releases a large amount of energy (ΔG°′ = –2840 kJ/mol); releasing it all at once would produce too much heat and harm the cell, so cells capture it gradually through enzyme-catalyzed steps.
How is energy from glucose oxidation stored in the cell?
Energy is trapped in small energy “quanta” like ATP or reduced electron carriers, which can then be used to power cellular processes.
Why are electron carriers used in biological systems?
Electron carriers are used to extract energy from oxidation reactions in manageable steps, allowing free energy to be captured as ATP instead of lost as heat.
What property must electron carriers have to function in biological redox reactions?
They must be able to exist in both oxidized and reduced forms to accept and donate electrons.
What are the main water-soluble electron carriers in biological systems?
NAD⁺, NADP⁺, FMN, and FAD.
What class of enzymes do NAD and NADP typically bind to?
NAD and NADP bind to dehydrogenases, which have a structure of alternating β-strands and α-helices.
What are the four main water-soluble electron carriers in biological systems?
NAD⁺ (nicotinamide adenine dinucleotide)
NADP⁺ (nicotinamide adenine dinucleotide phosphate)
FMN (flavin mononucleotide)
FAD (flavin adenine dinucleotide).
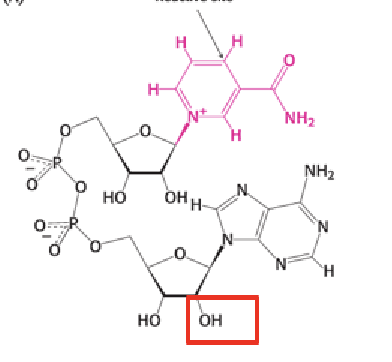
NAD
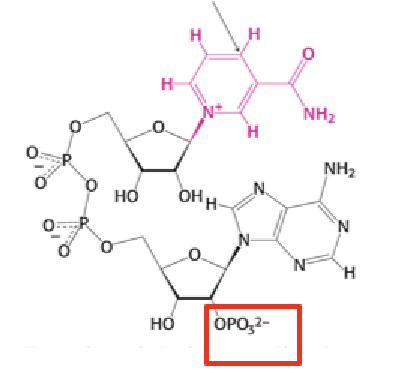
NADP
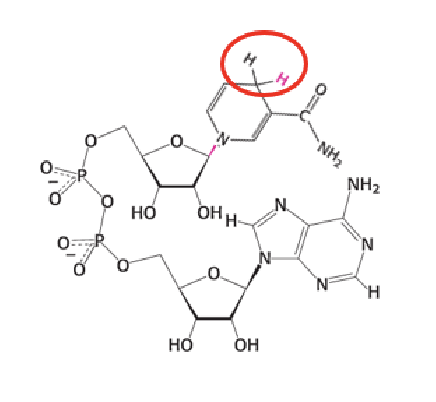
NADH
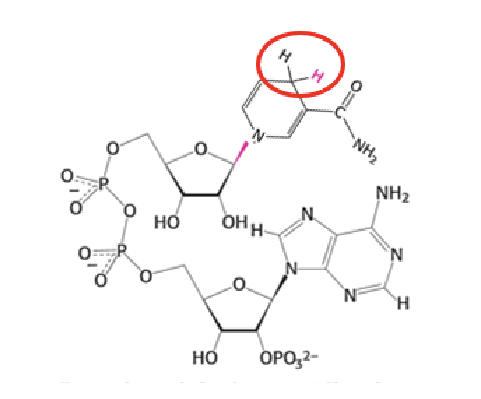
NADPH
What is the role of NAD⁺ in catabolism?
NAD⁺ accepts electrons (as a hydride ion) from molecules undergoing catabolic degradation, forming NADH.
What happens to NADH after it is formed in catabolism?
NADH donates its electrons to the mitochondrial electron transport chain to help generate ATP.
What is the main role of NADPH in metabolism?
NADPH supplies electrons for synthetic (anabolic) pathways.
How do NAD⁺ and NADP⁺ function as electron carriers?
They accept two electrons and one proton (a hydride ion) on the nicotinamide ring, forming NADH or NADPH.
Can NAD⁺ and NADP⁺ diffuse away from enzyme active sites?
Yes, both their oxidized and reduced forms readily diffuse away from enzyme active sites.
What is the standard reduction potential (E°′) for the NAD⁺/NADH redox pair?
E°′ = –0.32 V, meaning NADH is a strong electron donor. Standard reduction potential is low meaning NADH will readily give up its two electrons
How does electron flow occur between NADH and O₂ under standard conditions?
Electrons flow from NADH to O₂ because NADH has a lower E°′ (–0.32 V) and O₂ has a higher E°′ (+0.82 V), resulting in a favorable ΔE°′.
What is the standard free energy change (ΔG°′) for electron transfer from NADH to O₂?
ΔG°′ = –220 kJ/mol, calculated using ΔG°′ = –nFΔE°′ with n = 2 and F = 96.5 kJ/V·mol.
Why is ΔG°′ negative when electrons flow from NADH to O₂?
Because ΔE°′ is positive (+1.14 V), making ΔG°′ = –nFΔE°′ a large negative value, indicating a thermodynamically favorable reaction.
What distinguishes FMN and FAD from NAD⁺ and NADP⁺ as electron carriers?
FMN and FAD are tightly bound to enzymes and can accept or donate 2 electrons and 2 protons, unlike NAD⁺/NADP⁺ which are freely diffusible.
How can you recognize the reduced form of a flavin (FAD or FMN)?
All flavins have a 3-ring structure; the reduced form (FADH₂ or FMNH₂) has 2 added protons on the ring system.
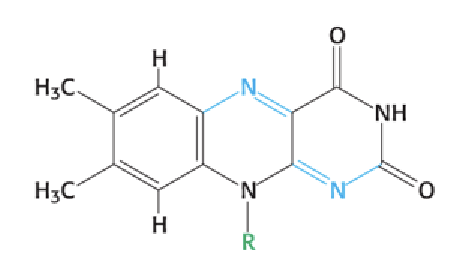
FAD
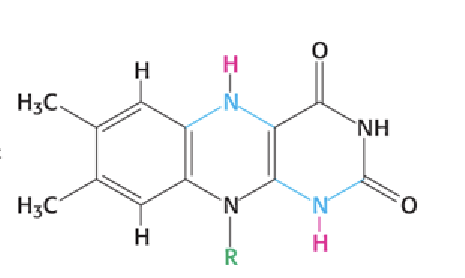
FADH2
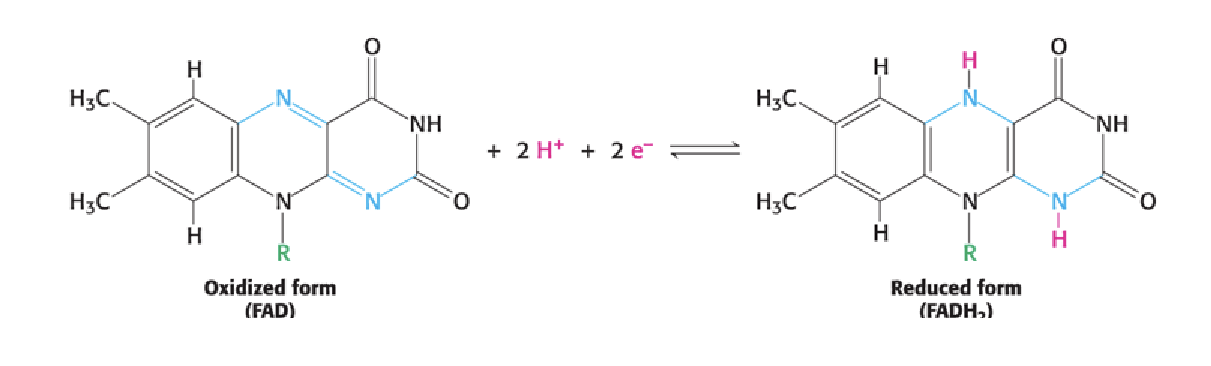
redox of FAD