Principles of Chemistry I Exam 2
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
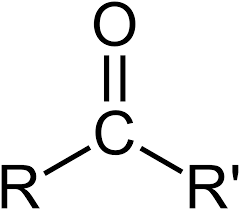
Ketone
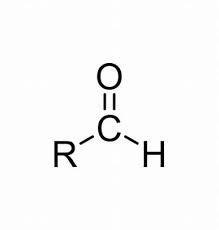
Aldehyde
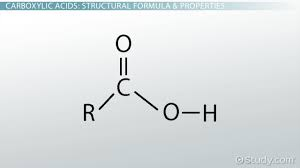
Carboxylic Acid
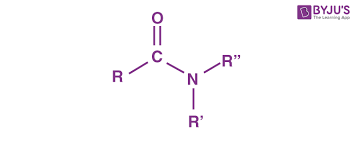
Amide
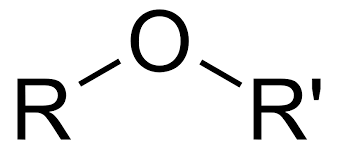
Ether
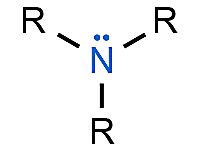
Amine
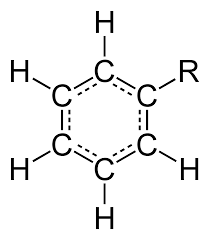
Phenyl
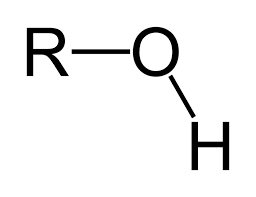
Alcohol
Arrange these elements from high to low electronegativity:
Al, Na, O, Cs, C
O, C, Al, Na, Cs
How are bond dipoles placed?
The arrow points from low to high electronegativity.
Which bond would you expect to be the most polar?
P-S
O-F
C-N
N-O
C-O
C-O
Specify the polarity of bonds and the molecule.
BeCl2
Polar bonds, nonpolar molecule.
Specify the polarity of bonds and the molecule.
OH2
Polar bonds, polar molecule
What does it mean to have partial positive character?
The atom with the lower electronegativity in a bond.
What is the wavelength, frequency, and energy of a pulse of light containing 100 photons?
1Wavelength, 1Frequency, 100Energy
True or false: It takes less energy to ionize the electron from the n=4 state than from the ground state.
True
True or false: The wavelength absorbed when the electron is excited from the ground state to n=4 is the same as the wavelength of light emitted when the electron falls from n=4 to the ground state.
True.
True or false: On average, the electron is closer to the nucleus in the n=4 state than in the n=1 state.
False.
R
True or false: The wavelength of light emitted when the electron falls from n=4 to n=2 is shorter than the wavelength of light emitted when the electron falls from n=4 to n=1.
False.
True or false: The n=4 excited state is the first possible excited state.
False.
If a solution appears orange, then the compound in the solution…
Absorbs blue light.