The Development of the Atomic Model (SHE TASK 2)
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Democritus (4th Century BC)
Believed:
different substances had Atomos (invisible particles) of different sizes & shapes.
atoms couldn’t be broken down any further
that atoms exist in empty space, and this allows them to move

John Dalton (1808)
all matter is made of atoms
atoms of an element are identical
each element has different atoms
atoms of different elements combine in constant ratios to form compounds
atoms are rearranged in reactions
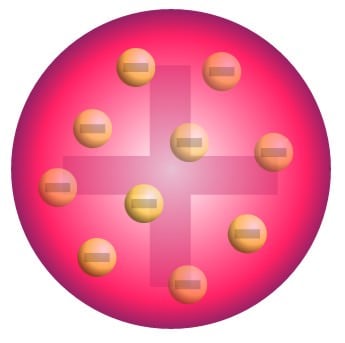
Joseph John Thomson (1897)
Cathode Ray Tube which had a noble gas, a + & - electrodes
gas particles attracted to + electrodes
particles must have a - charge (electrons)
“Plum Pudding Model” = atom was a + sphere with electrons shoved into the sides of it.
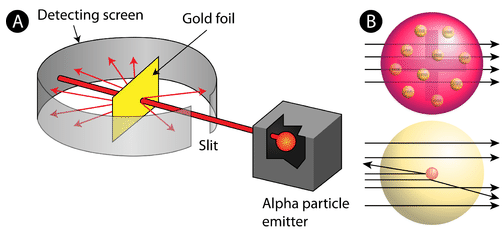
Ernest Rutherford (1910)
Gold Foil Experiment = shot high energy beam of + alpha particles into gold foil
Expected: the alpha particles would pass straight through the foil, but:
few particles were deflected at small angles
very few particles were deflected at large angles
Proved that all atoms are mostly empty spaced
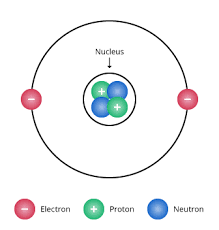
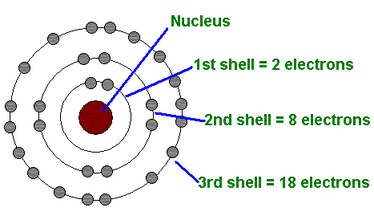
Niels Bohr (1913)
electrons revolve around the nucleus in fixed, circular orbit
electrons’ orbits correspond to specific energy levels or shells in the atom
electrons only occupy fixed energy levels and can’t exist between two energy levels
electron orbits of larger radii correspond to energy levels of higher energy

James Chadwick (1932)
conducted experiments bombarding (forcefully hitting) beryllium atoms with alpha particles (2 protons & 2 neutrons)
discovered neutrons (its mass is similar to protons, but it has no electrical charge)