Carboxylic Acid Derivatives
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Order these in carbonyl carbon reactivity: amides, acid chlorides, aldehydes, esters, ketones, carboxylic acids.
The order of carbonyl carbon reactivity from highest to lowest is acid chlorides > aldehydes > ketones > esters > carboxylic acids > amides.
If you only had a Grignard reagent, how can you make a carboxylic acid? Furthermore, if you had an alkyl halide, how can you make the same thing?
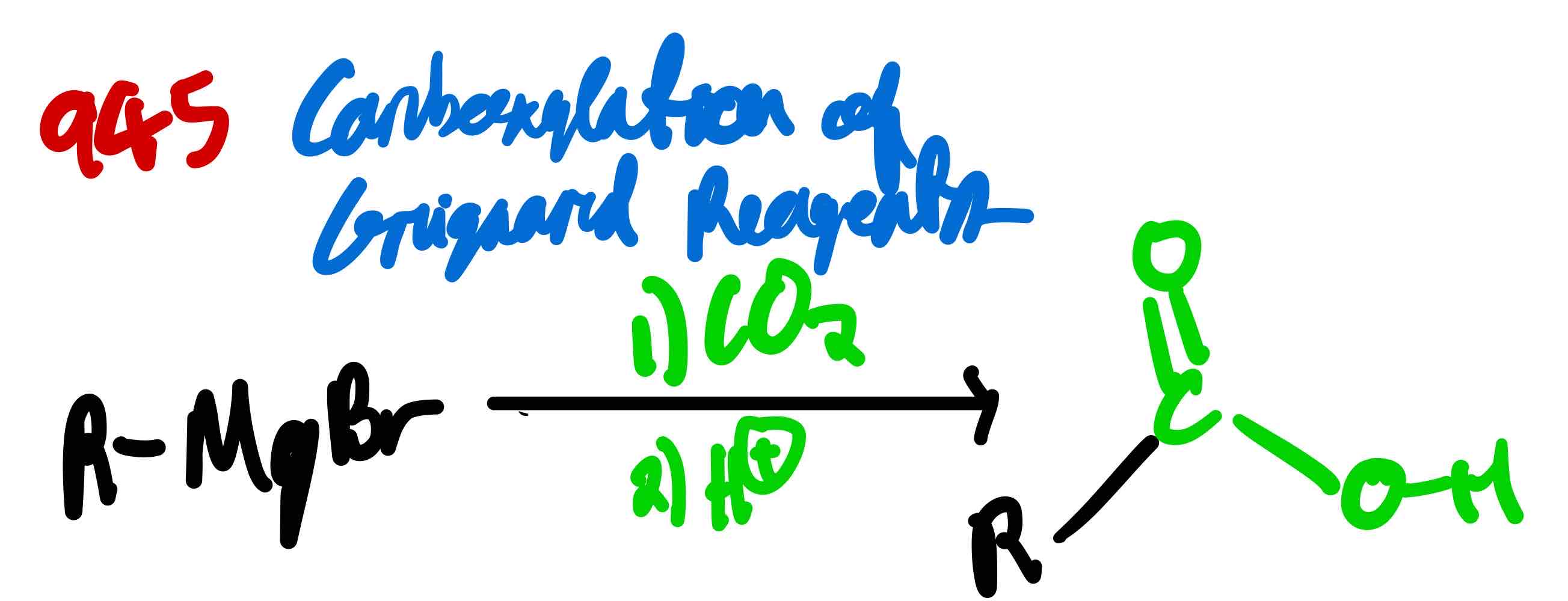
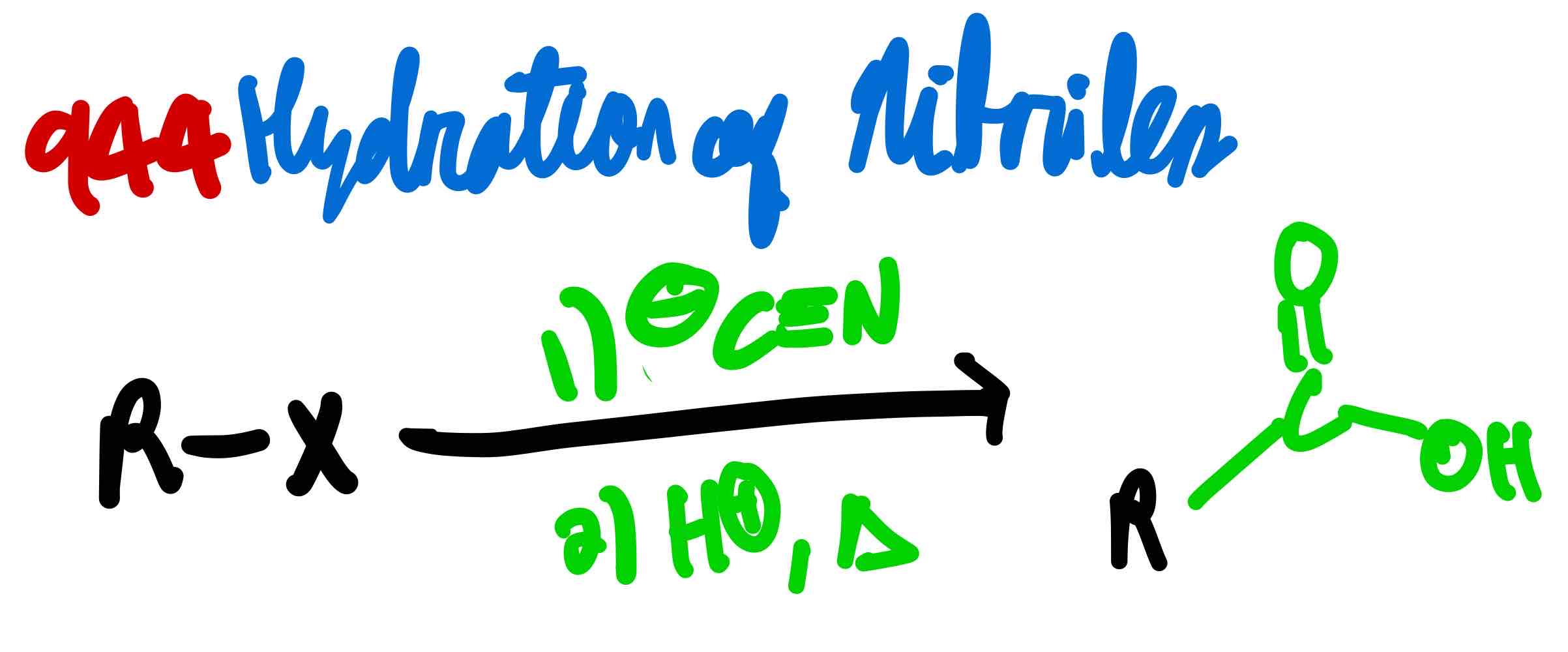
If you had a methyl or primary alkyl halide, how can you make a carboxylic acid?
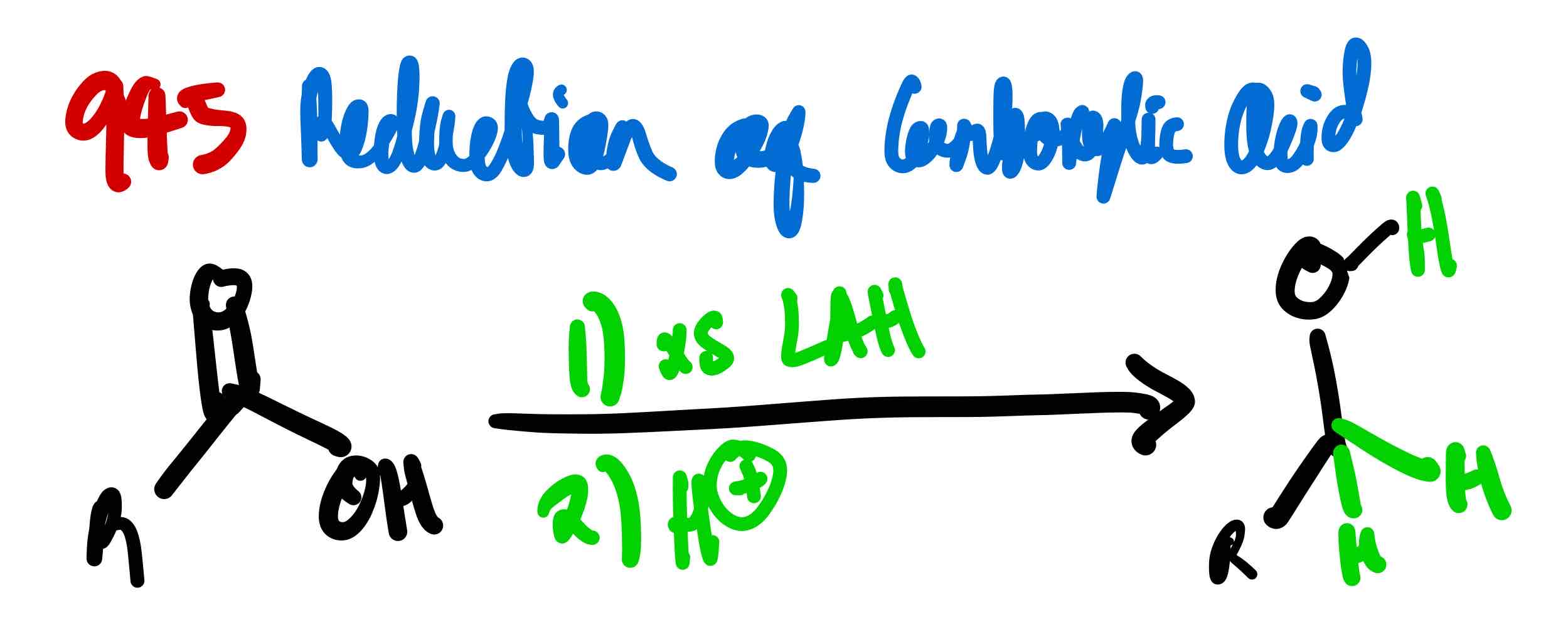
If you wanted to turn a carboxylic acid to a primary alcohol, how would you proceed?
If you wanted to convert a carboxyl to a primary hydroxy when another carbonyl-containing group is present on a compound, how would you proceed?
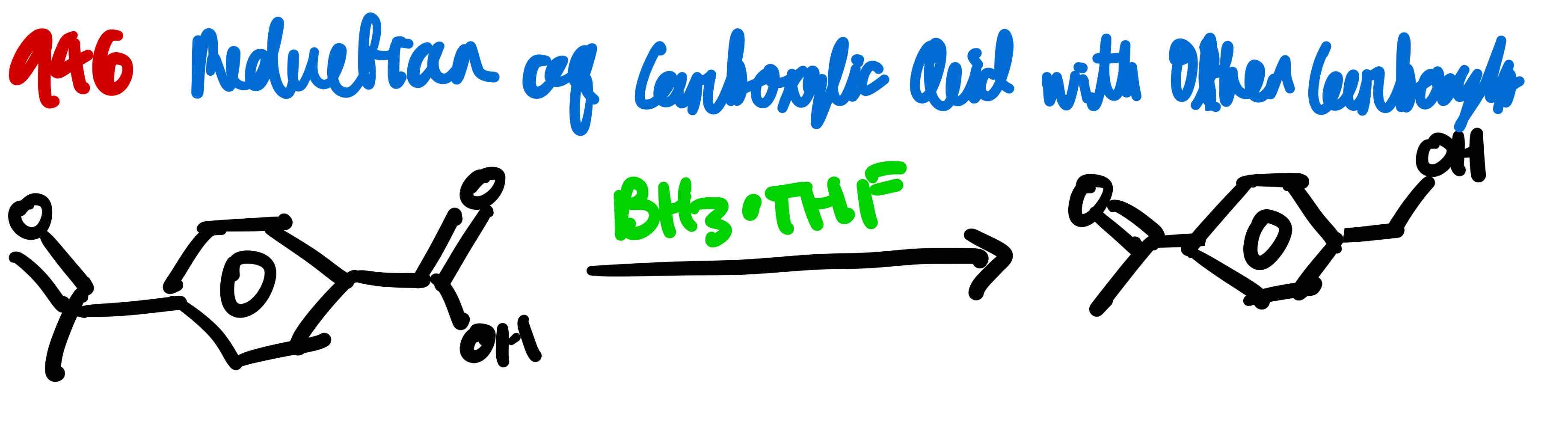
From carboxylic acid to acid chloride, how?

From acid chloride to carboxylic acid, how?
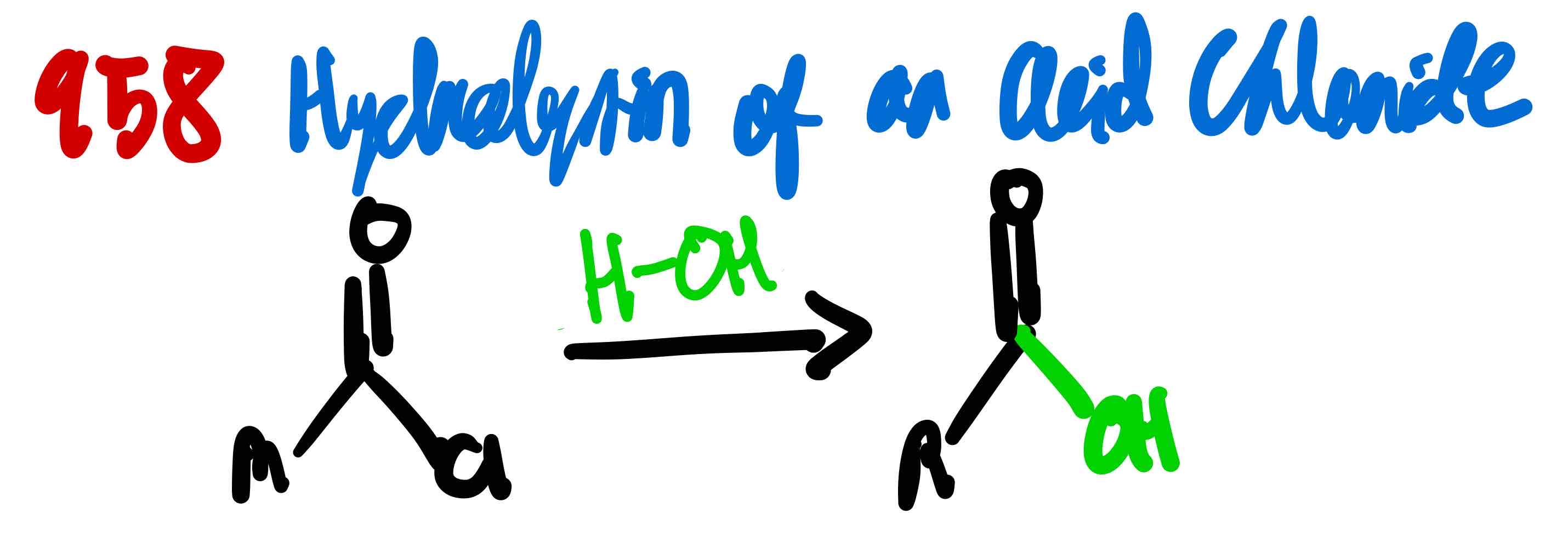
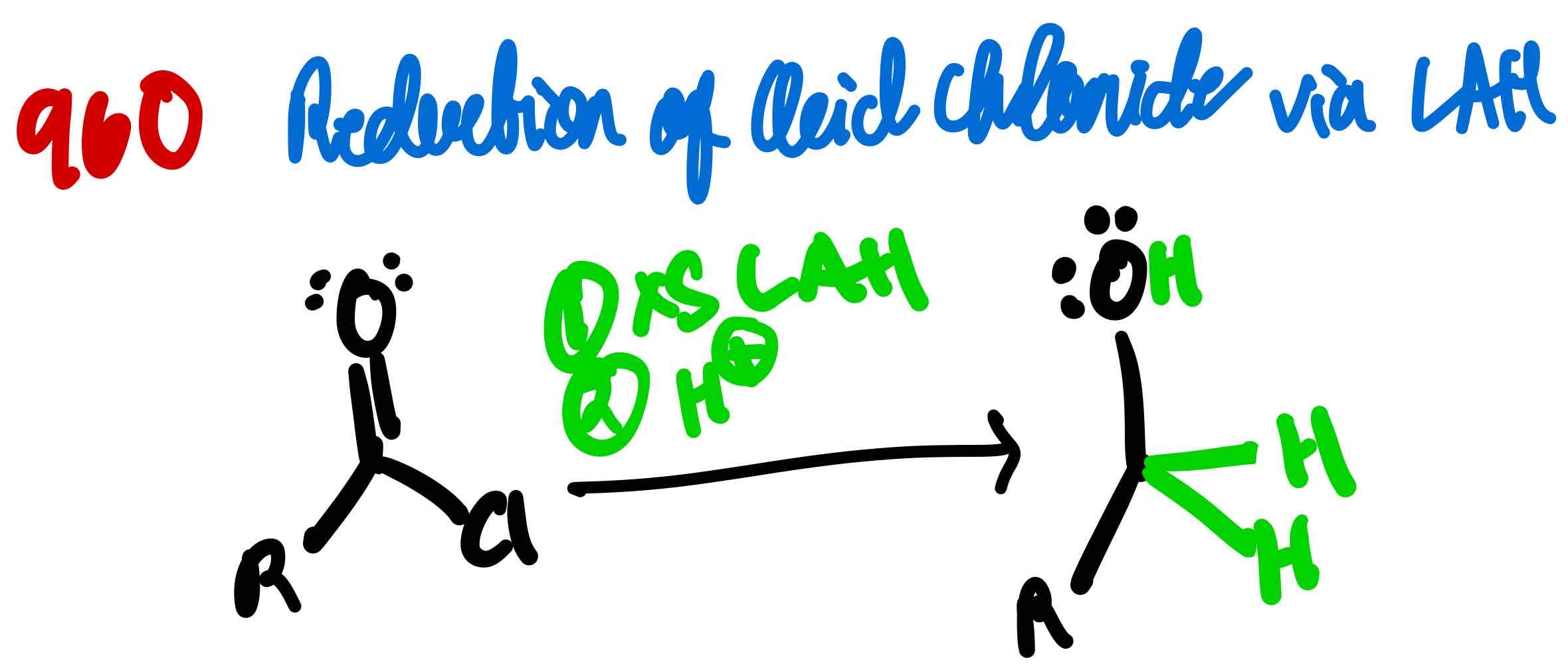
From acid chloride to primary alcohol, how?
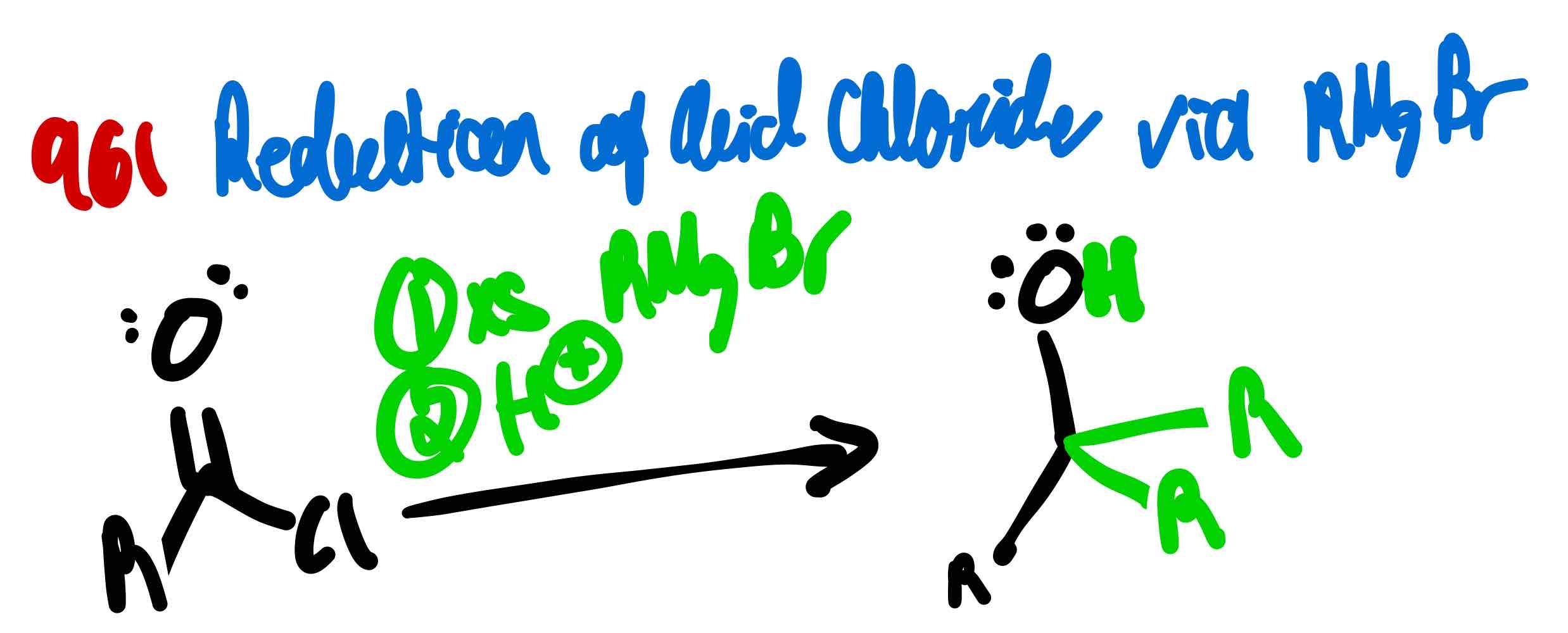
From acid chloride to tertiary alcohol, how?
From acid chloride to aldehyde, how?

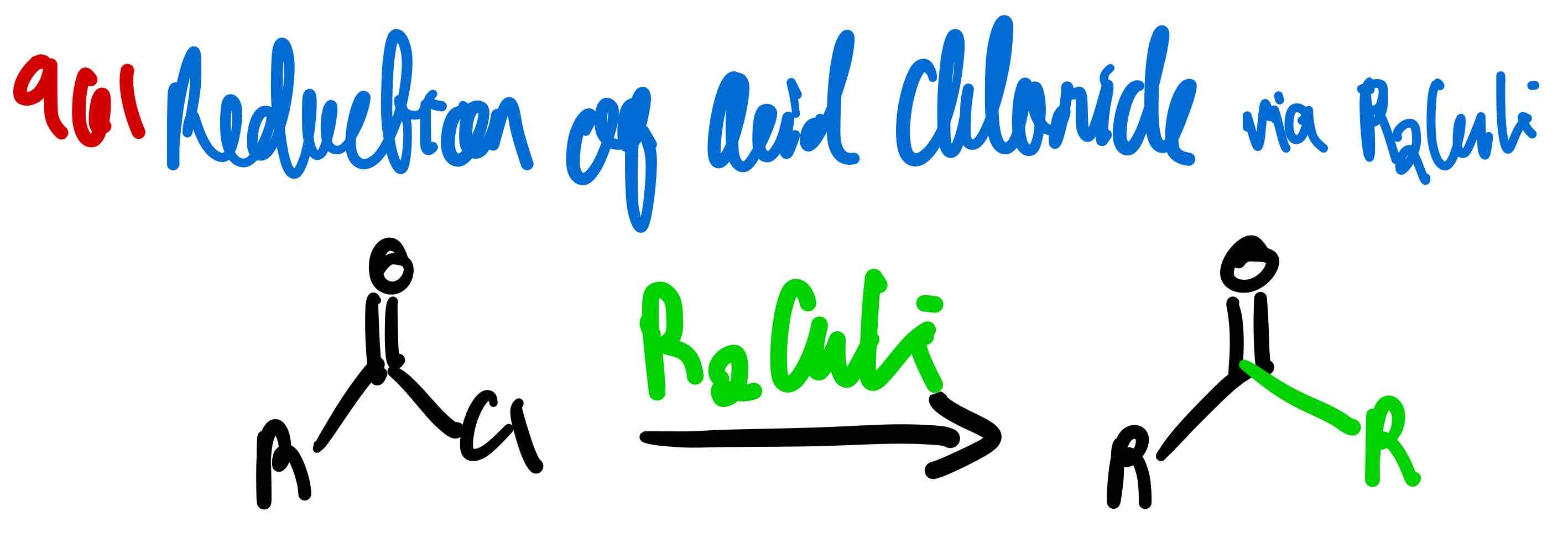
From acid chloride to ketone, how?
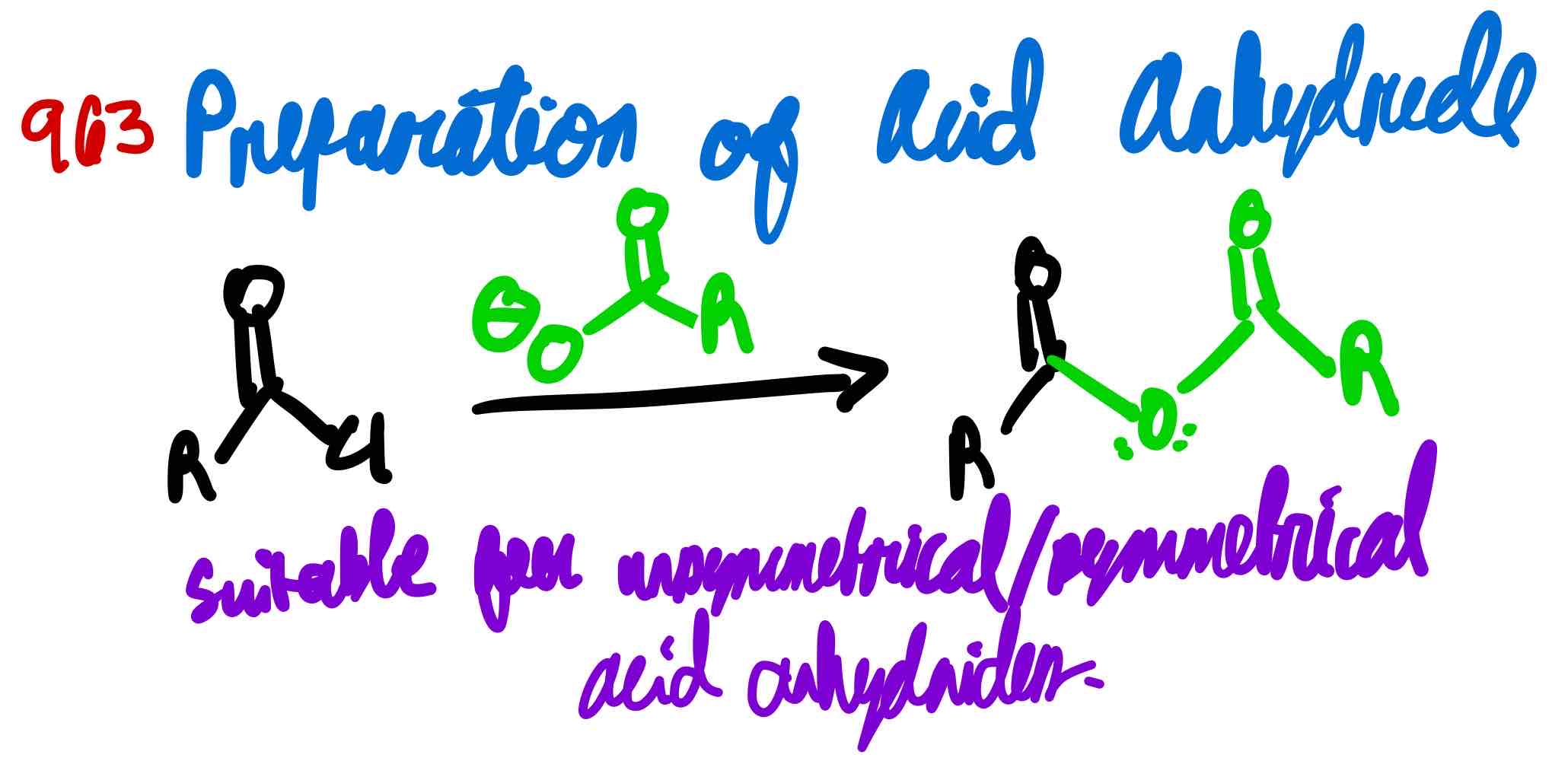
From acid chloride to acid anhydride, how?
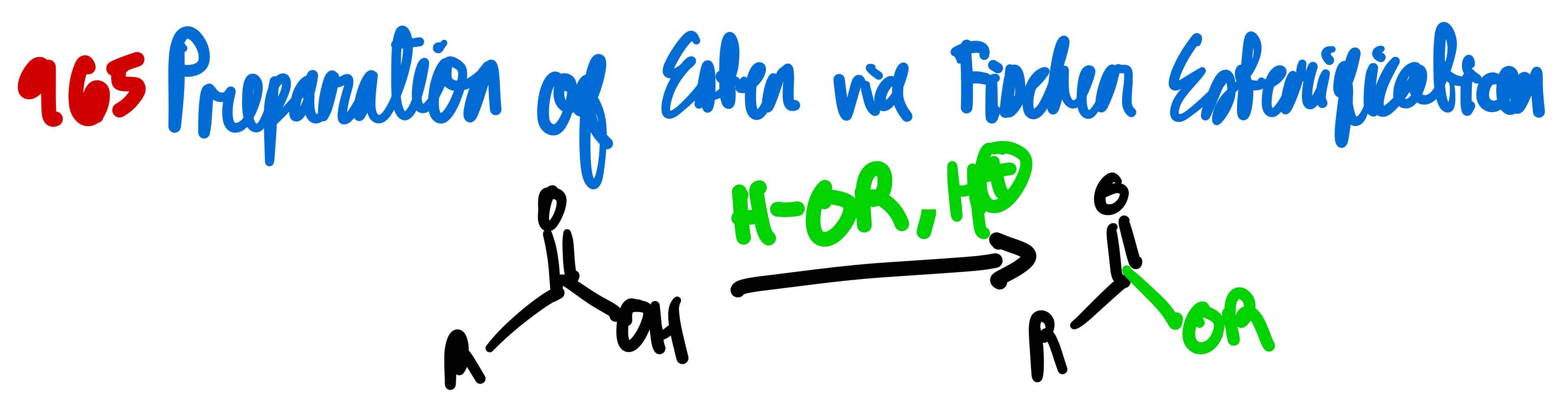
From carboxylic acid to ester, how?
From acid chloride to ester, how?
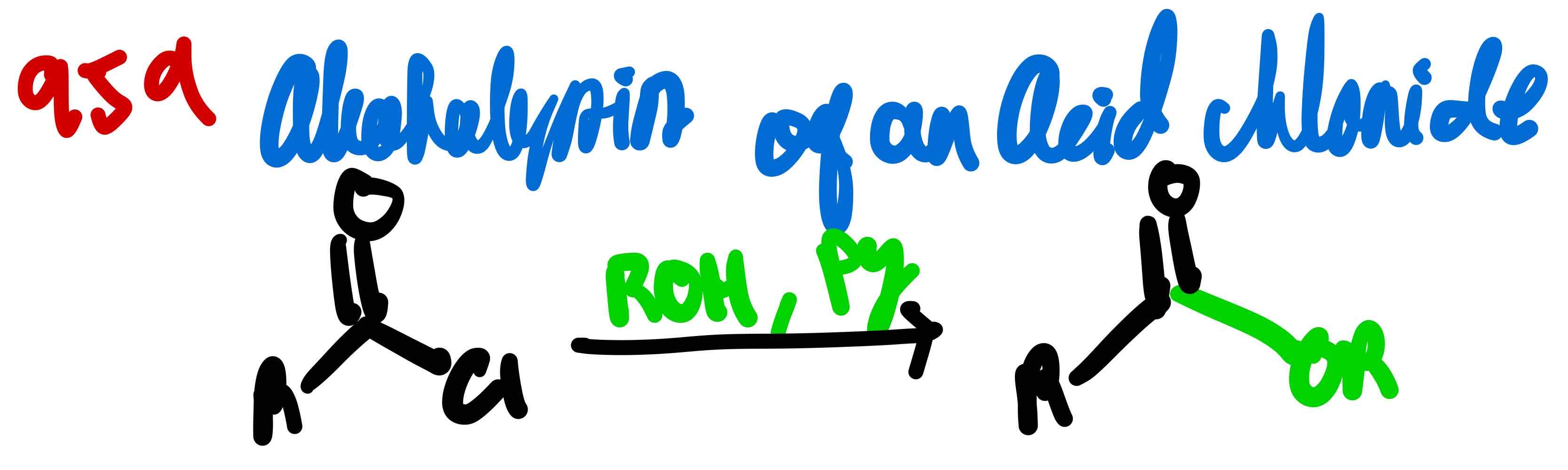
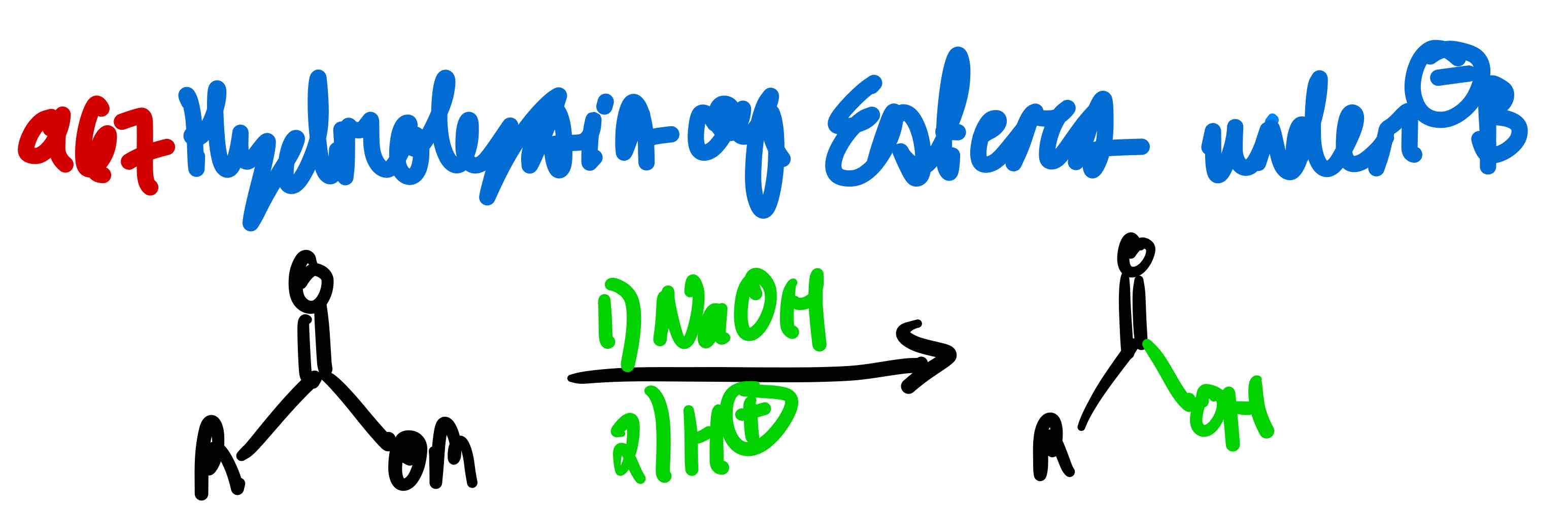
From ester to carboxylic acid in basic conditions, how?
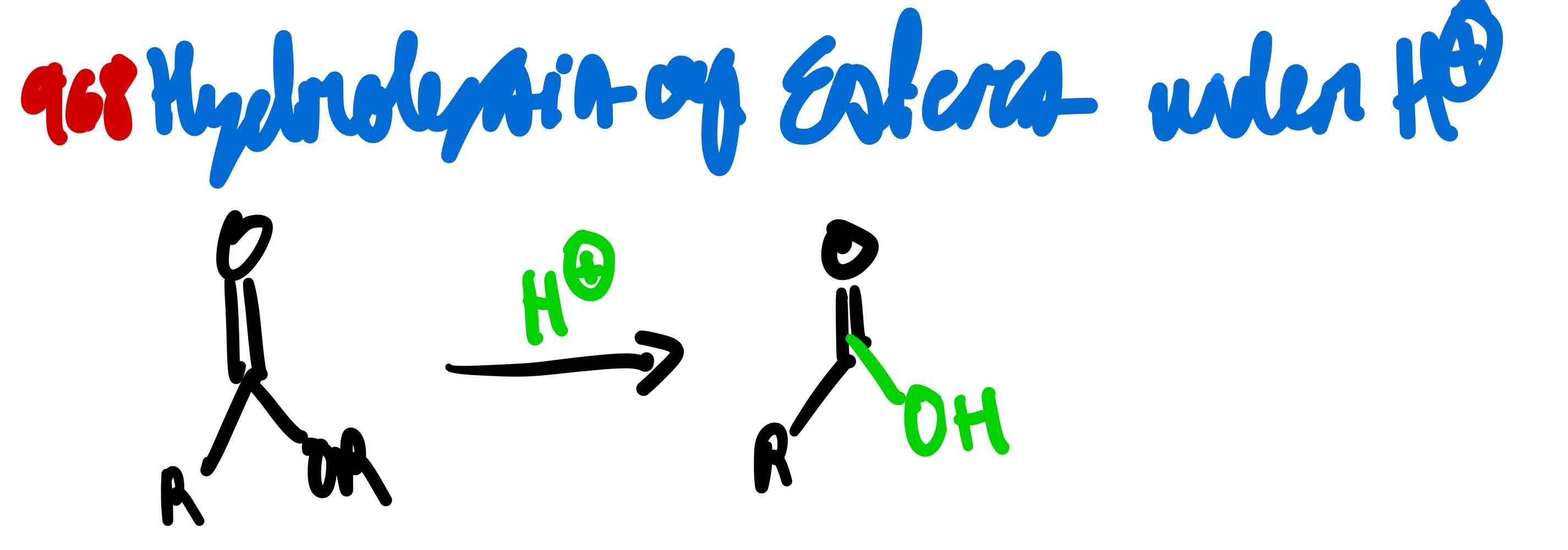
From ester to carboxylic acid in acidic conditions, how?
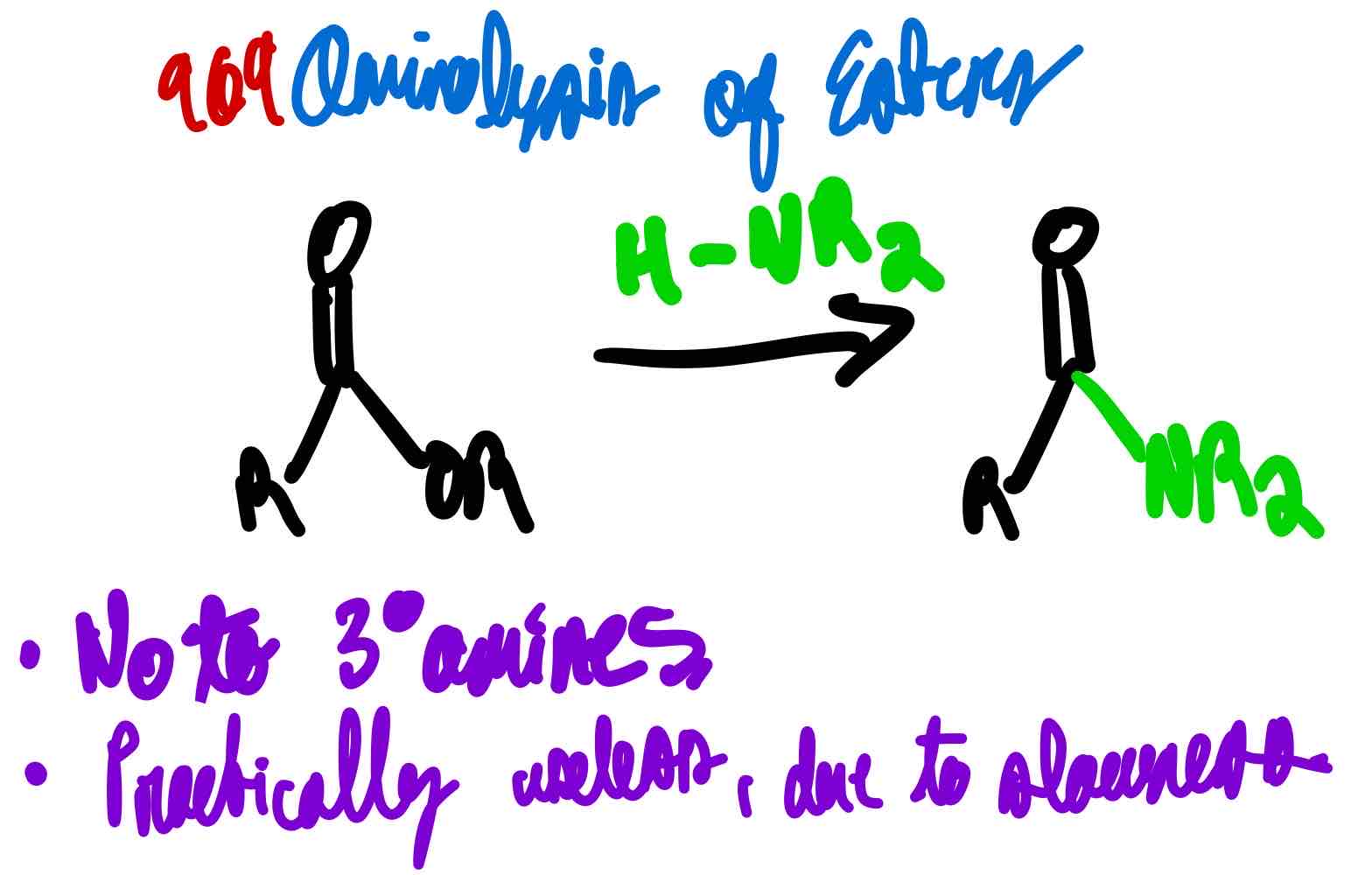
From ester to amide, how?
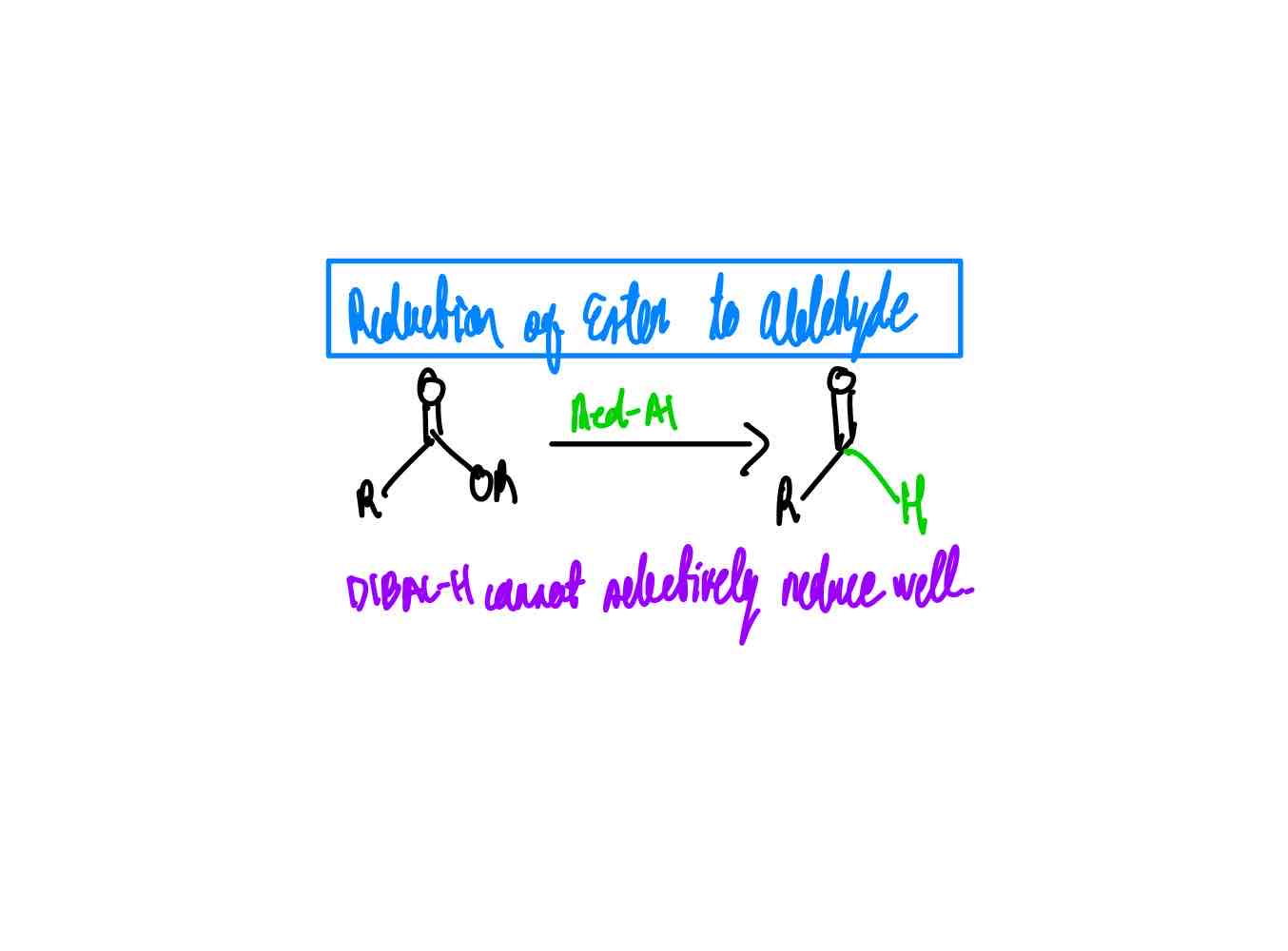
From ester to aldehyde, how?
Why can’t we use DIBAL-H?
What does Red-Al look like?
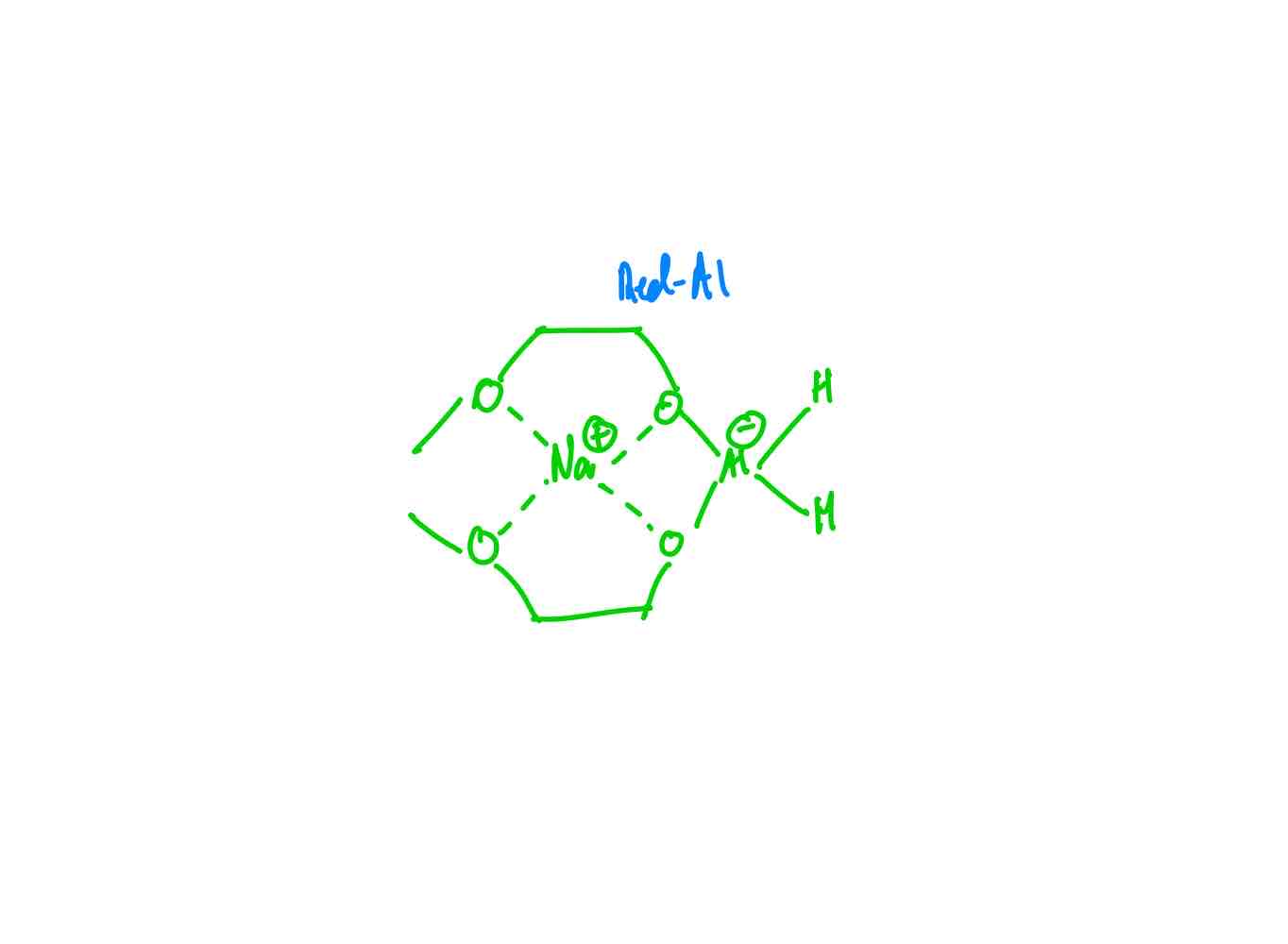
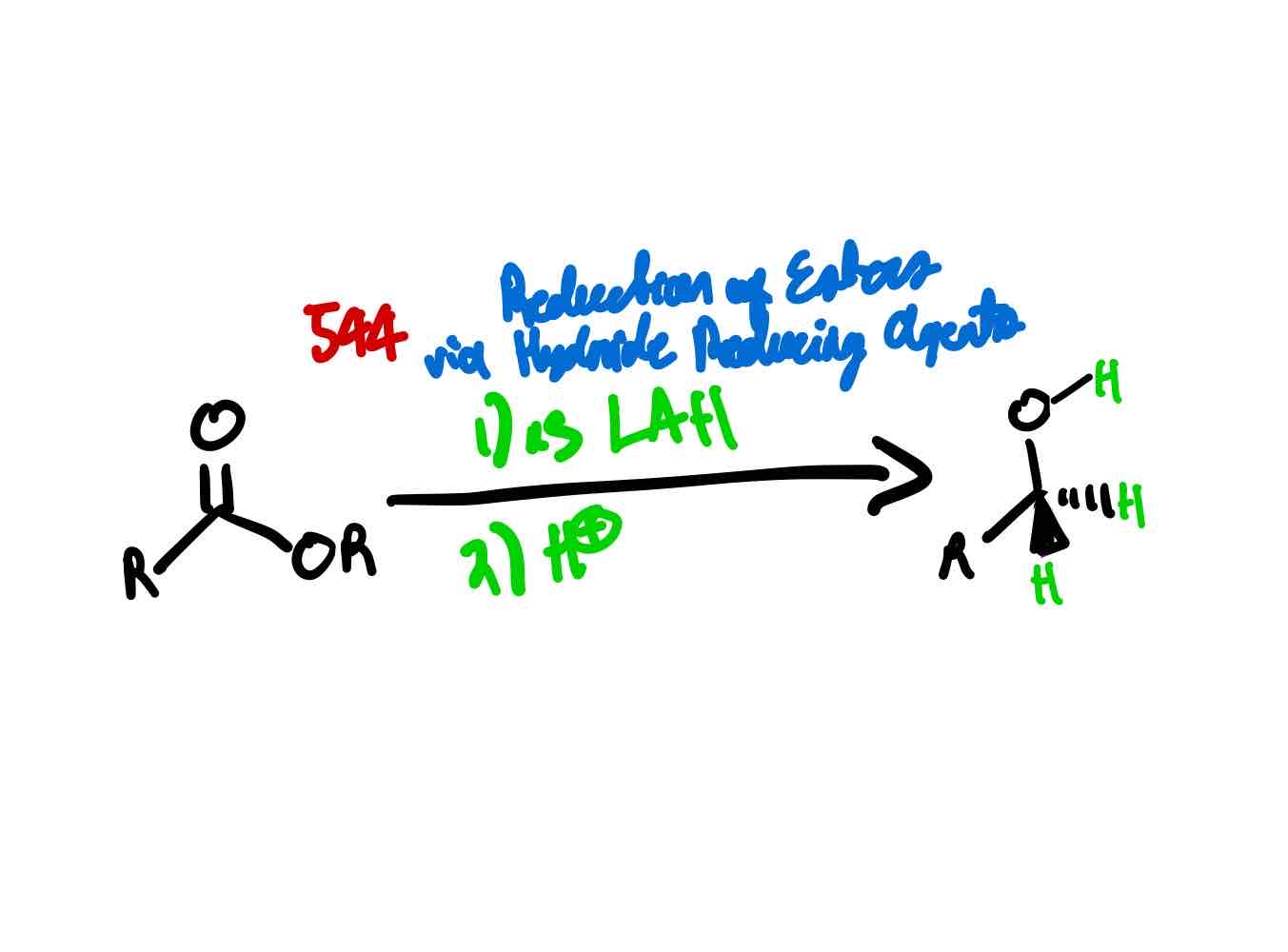
If you wanted to convert an ester to a primary alcohol by:
Converting the carbonyl to a hydroxy
Installing two hydridos to the carbonyl carbon
How would you proceed?
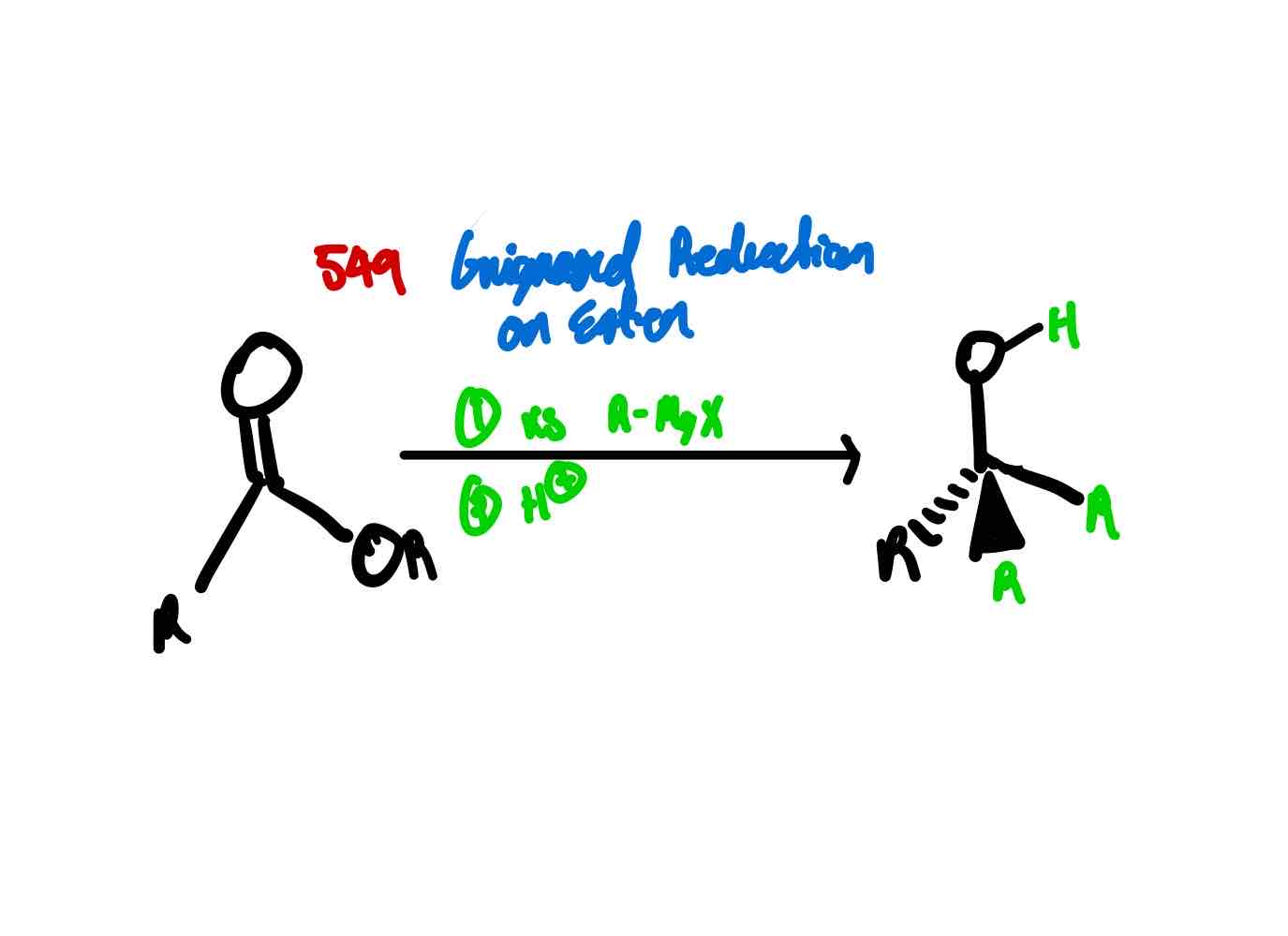
If you wanted to convert an ester to a tertiary alcohol by:
Converting the carbonyl to a hydroxy
Installing two R groups to the carbonyl carbon
How would you proceed?
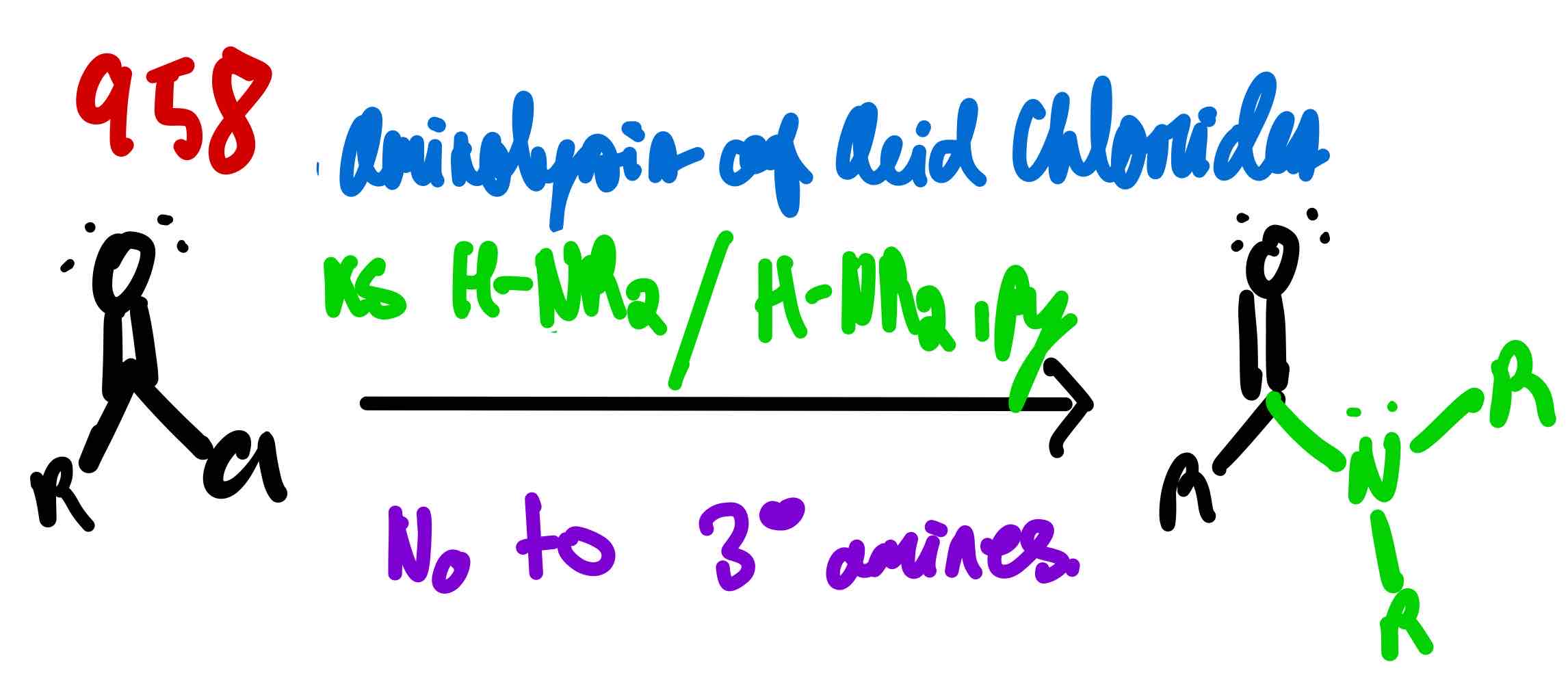
From acid chloride to amide, how?
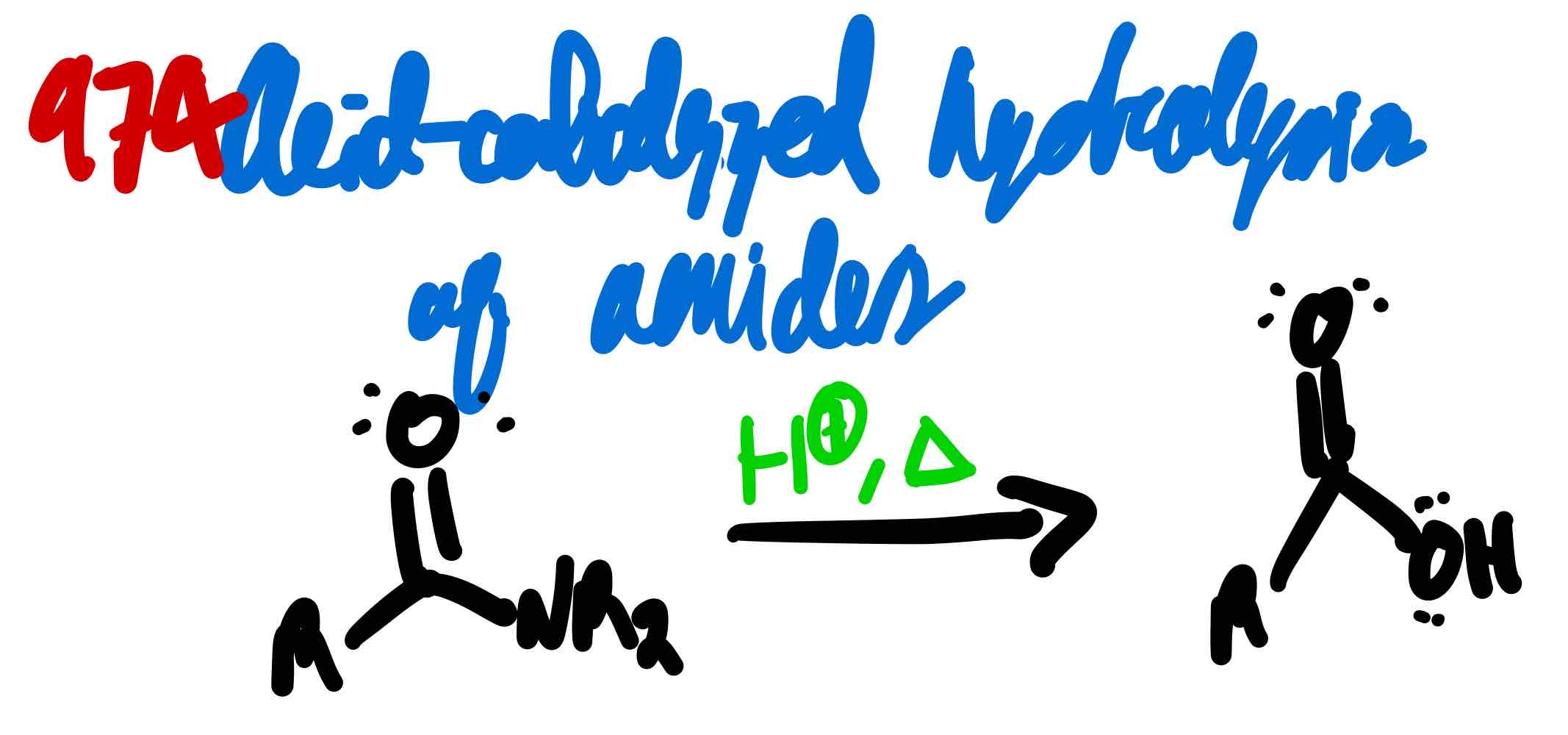
From amide to carboxylic acid in acidic conditions, how?
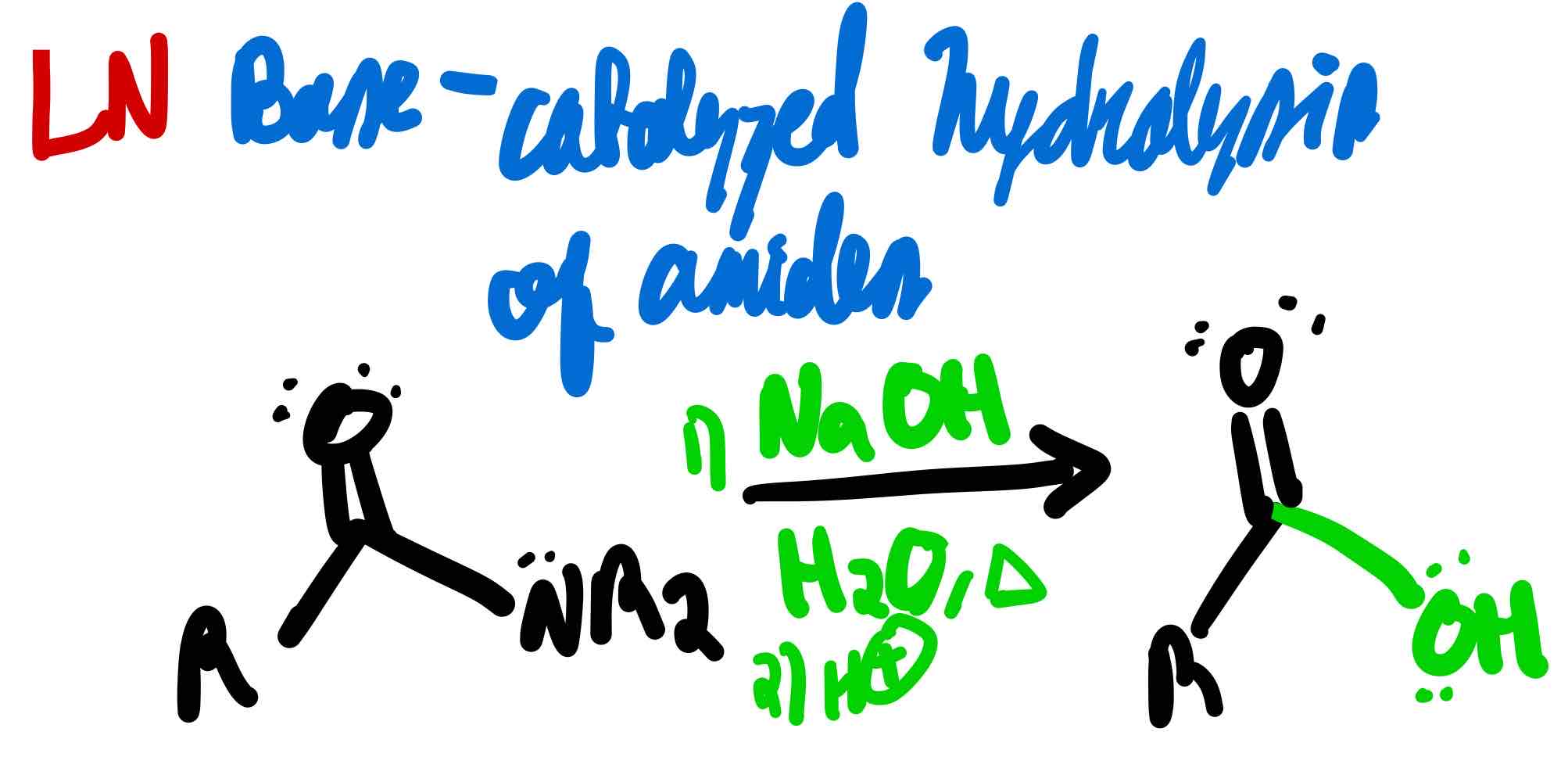
From amide to carboxylic acid in basic conditions, how?
From amide to amine, how?
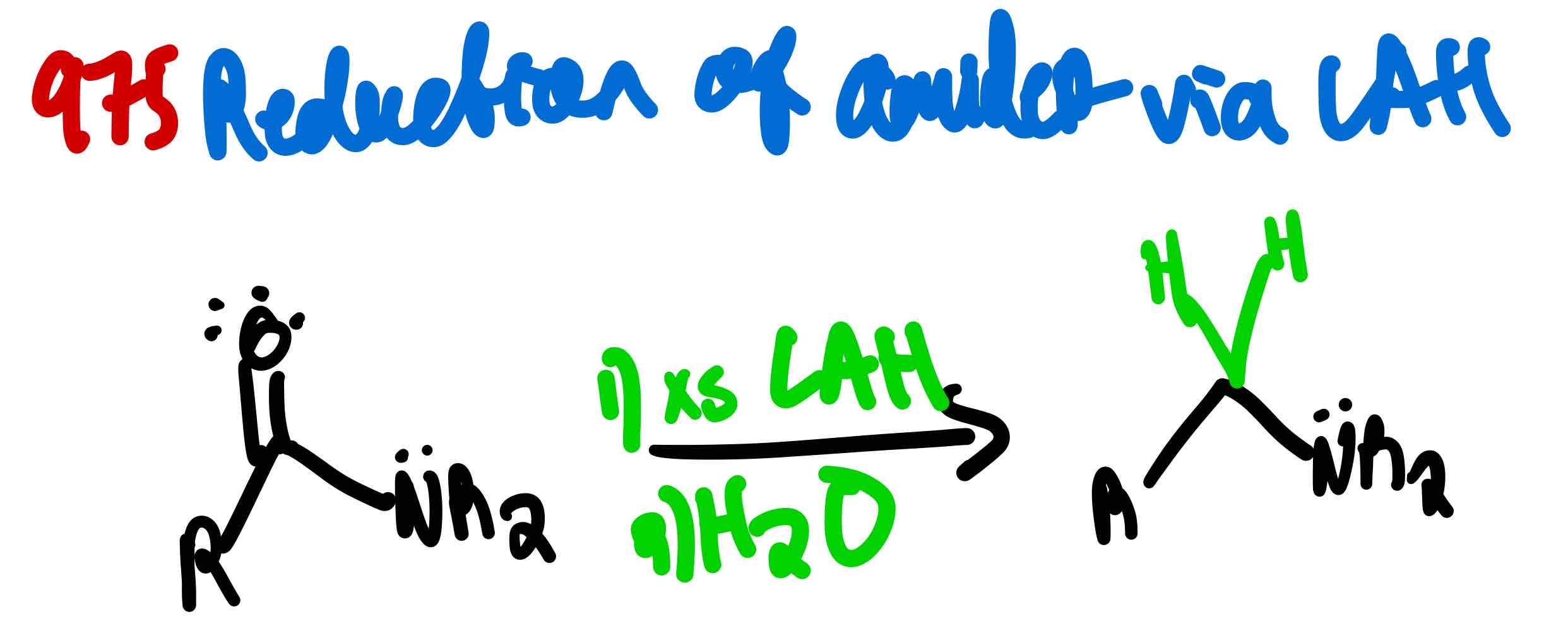
If you had a molecule containing carbonyls and one of these carbonyls belong to an amide, how would you proceed to reduce the amide to an amine?
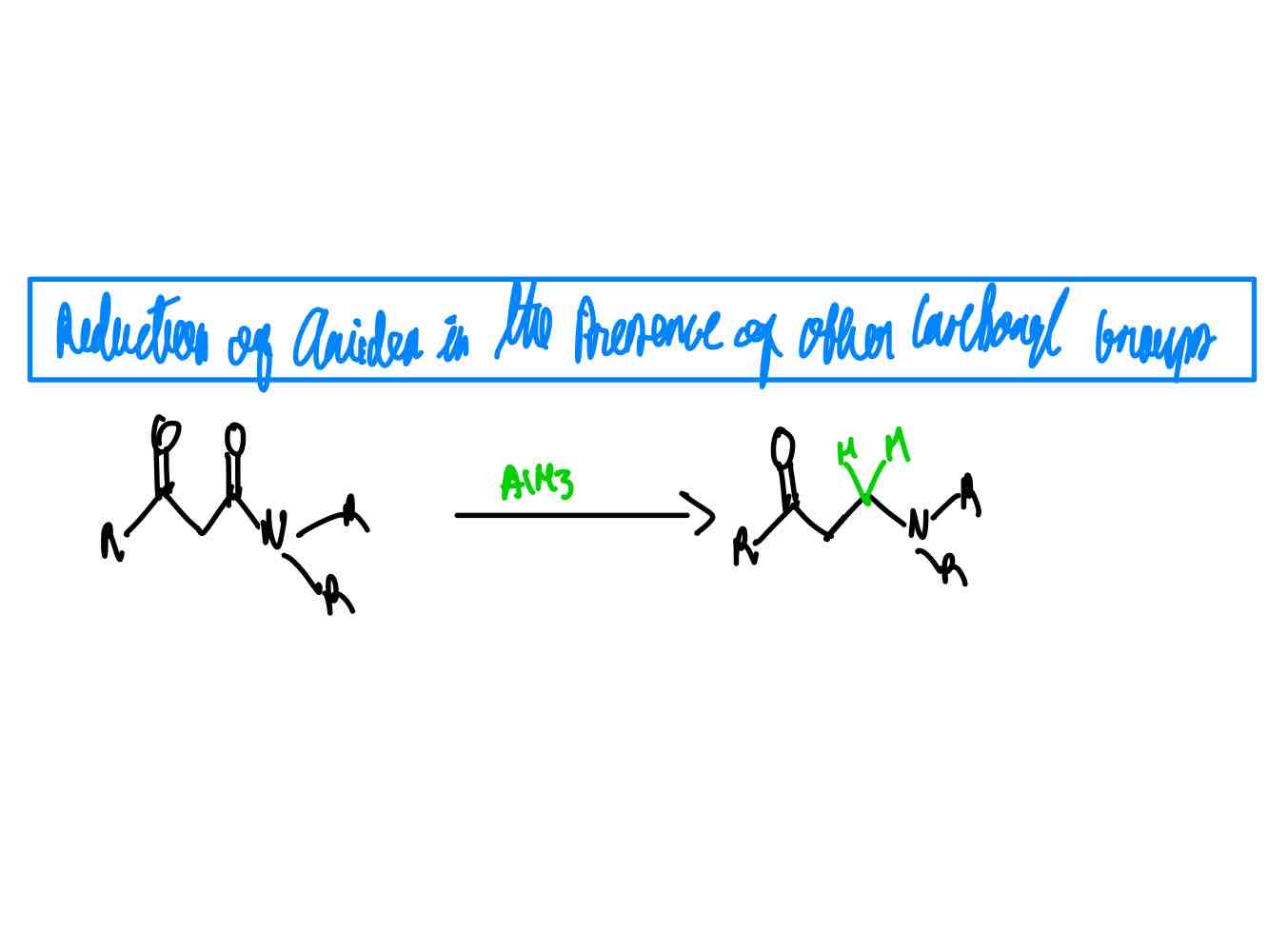
For the reduction of amides to amines in the presence of other carbonyl-containing groups:
Why can’t we use LiAlH4?
Why can’t we use Clemmensen, Wolff-Kishner, and desulphurization for this?
What’s the mechanism of this reaction similar to?

From amide to nitrile, how?
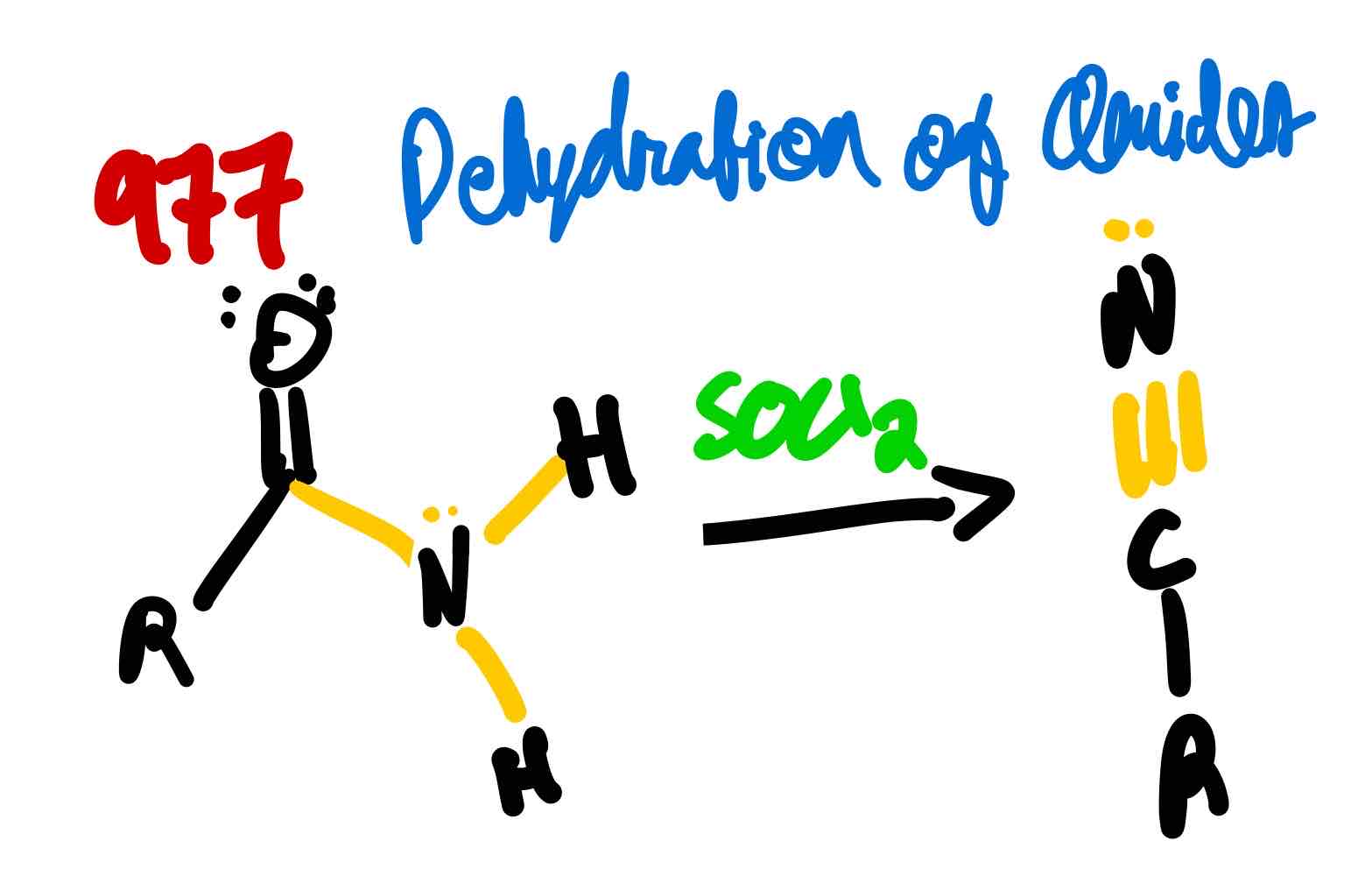
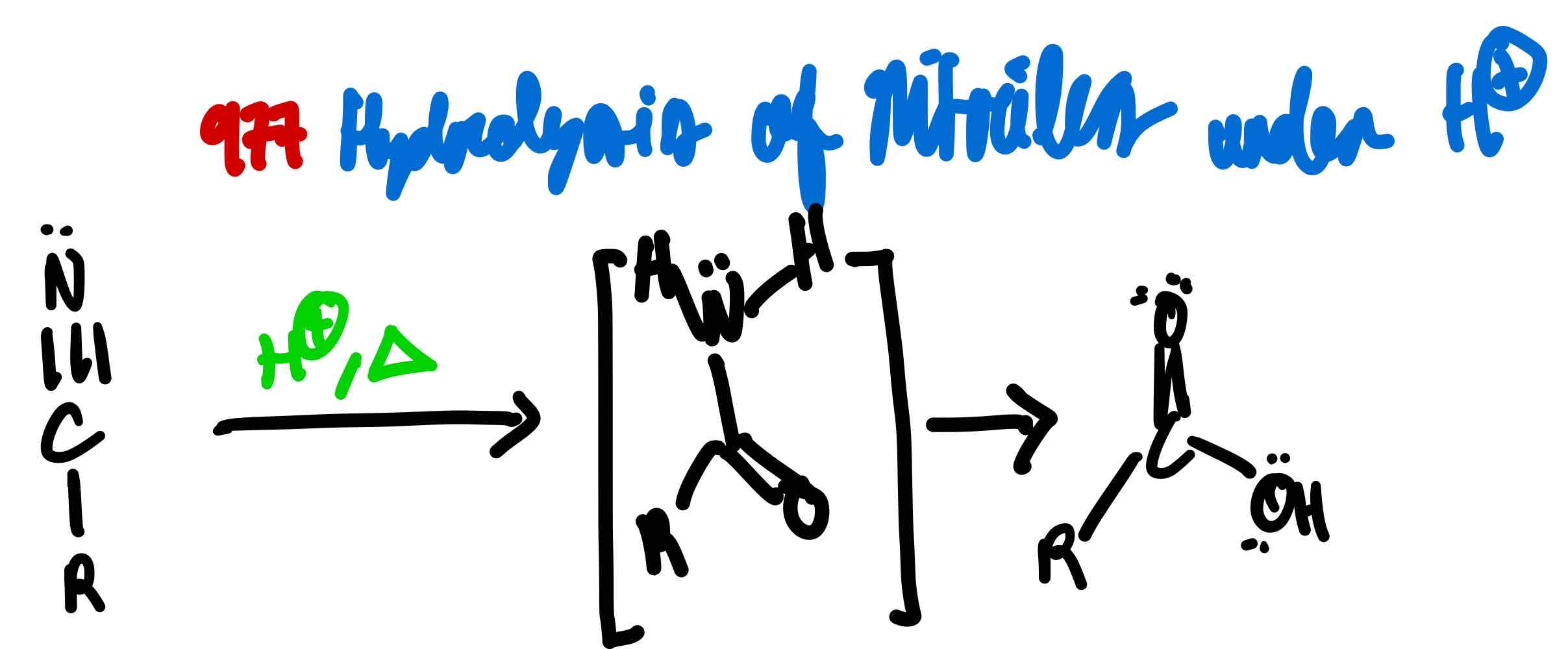
From nitrile to carboxylic acid in acidic conditions, how?
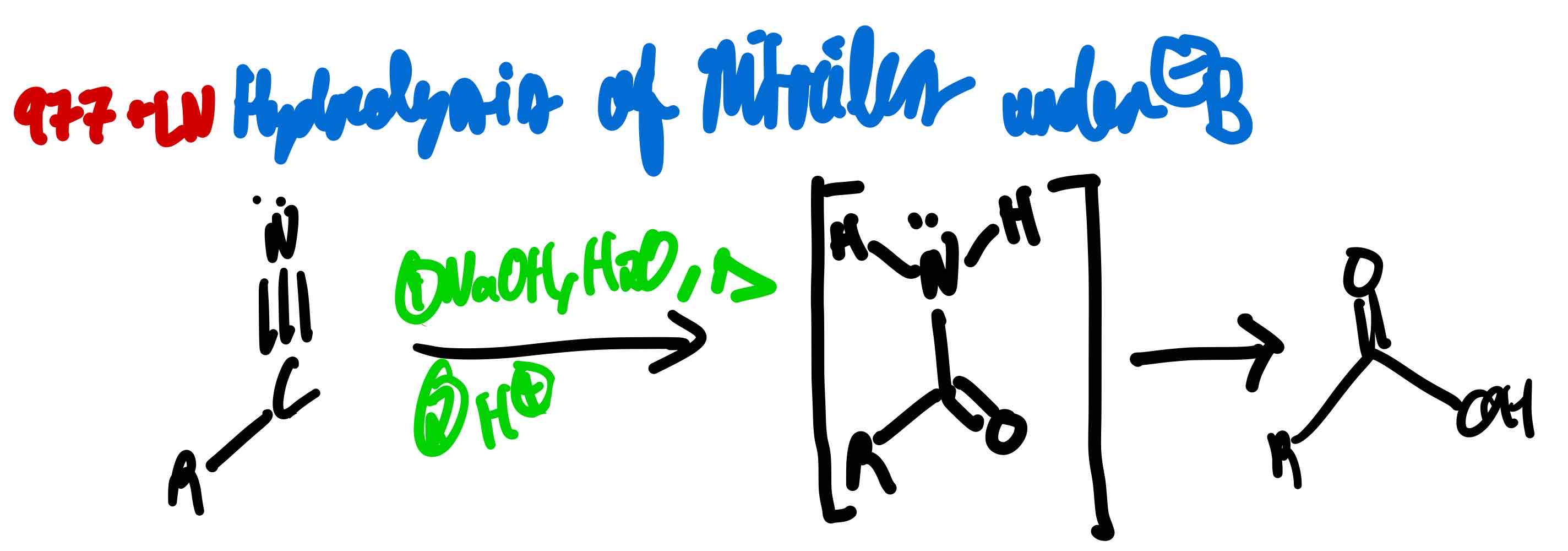
From nitrile to carboxylic acid in basic conditions, how?
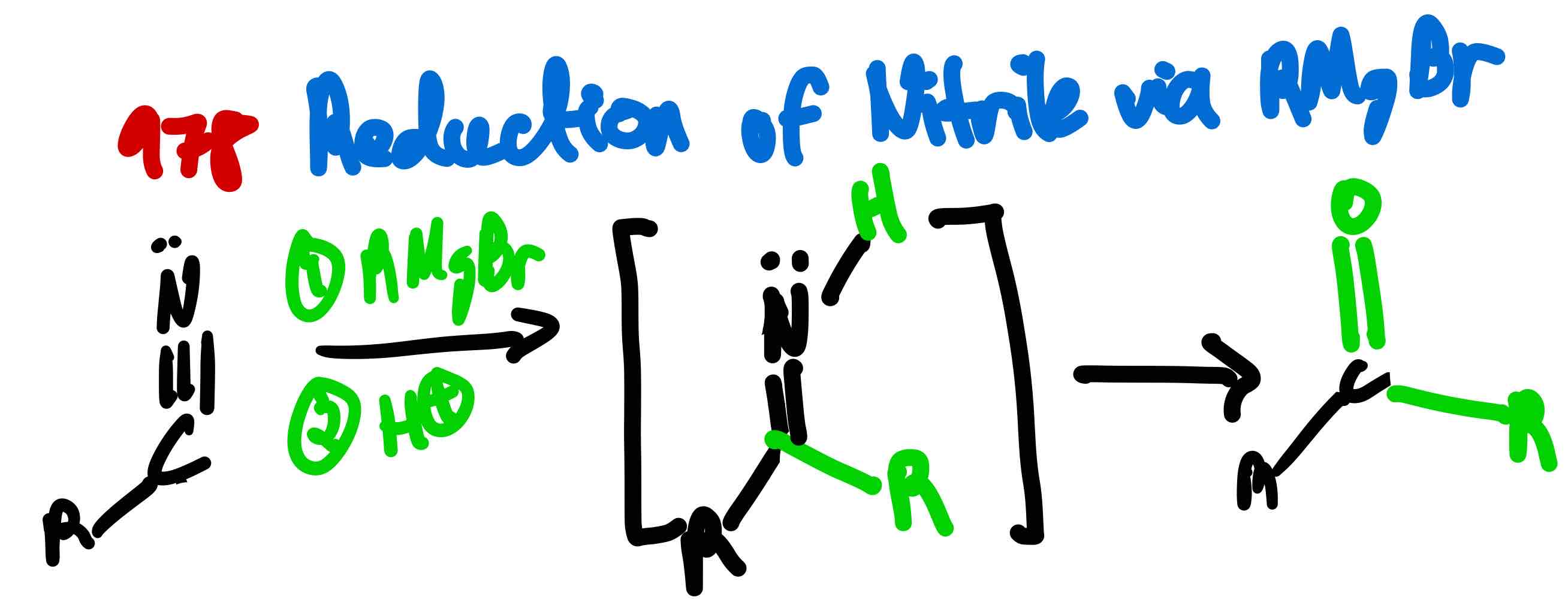
From nitrile to ketone, how?
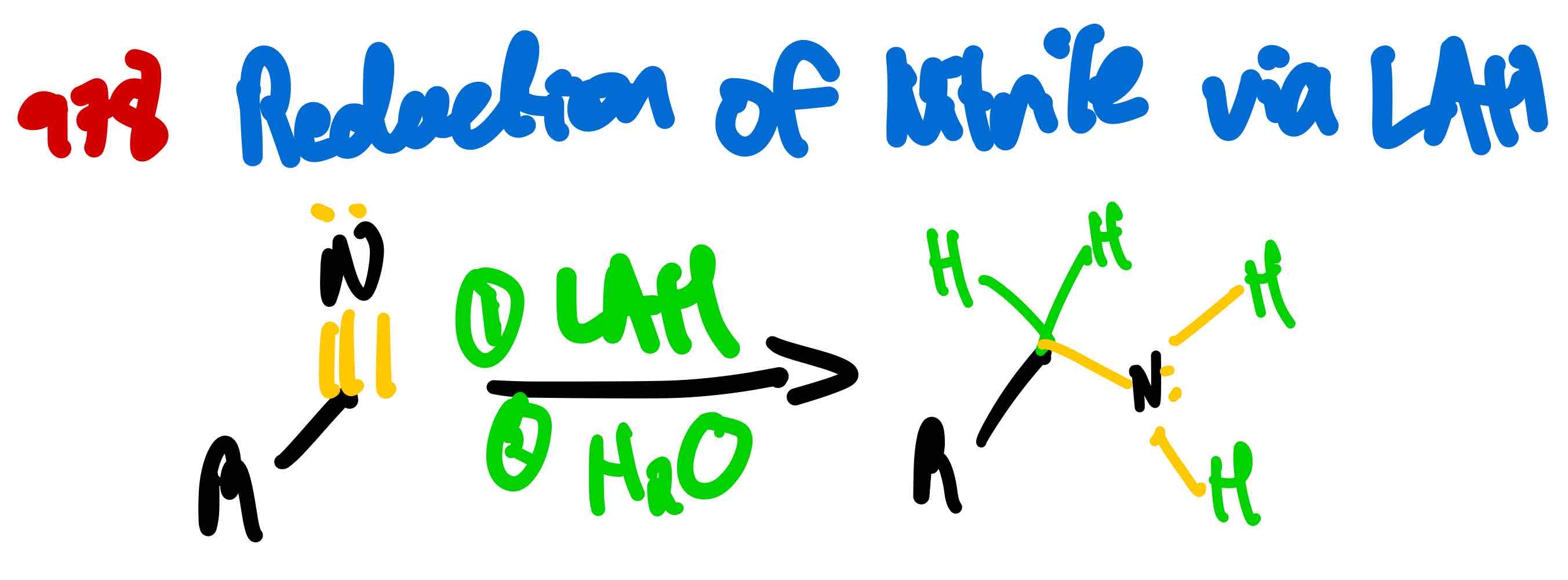
From nitrile to amine, how?
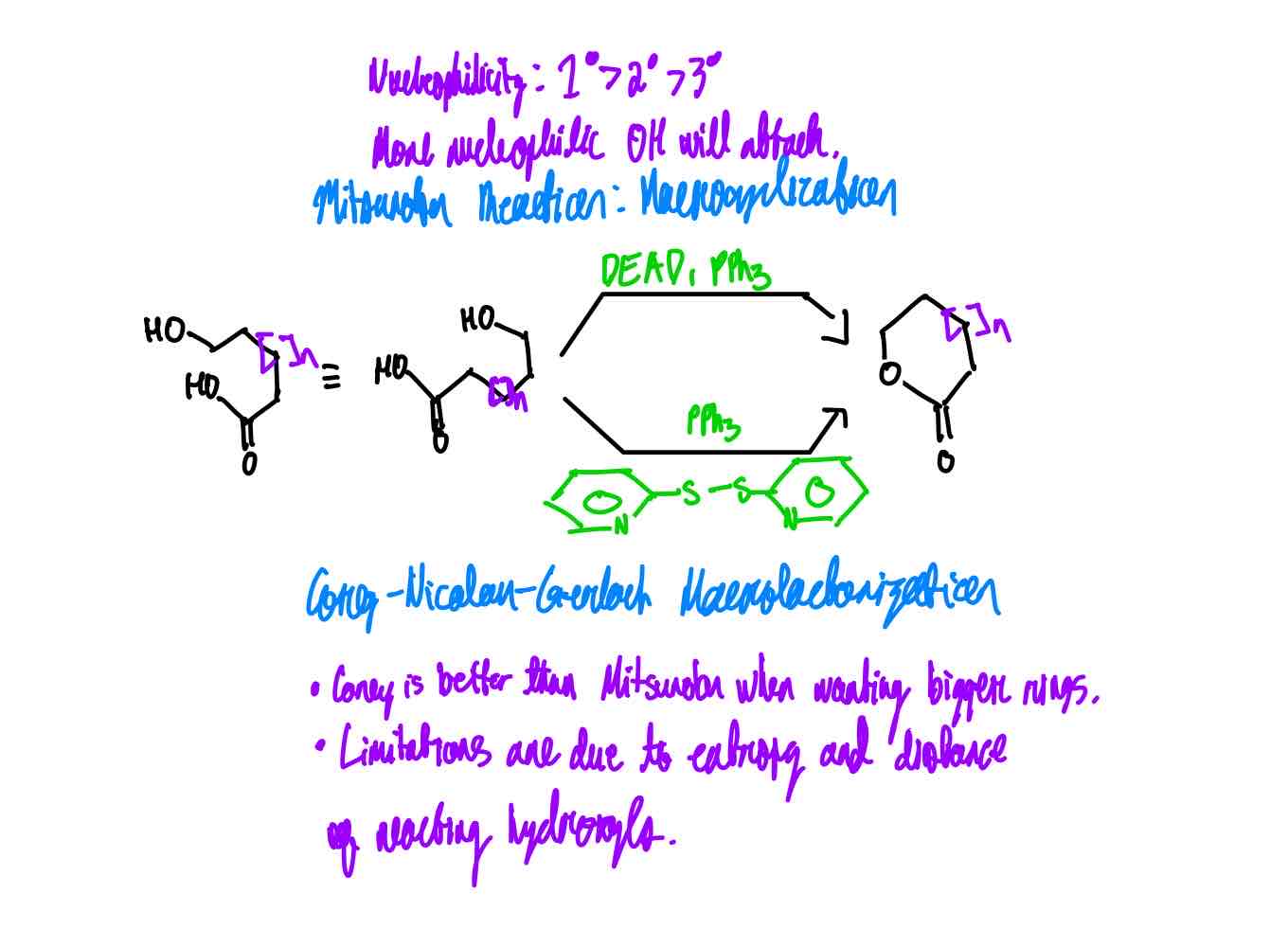
If you were to make a macrocycle from hydroxy carboxylic acids, what are the two ways that you could proceed? What’s to note about either?
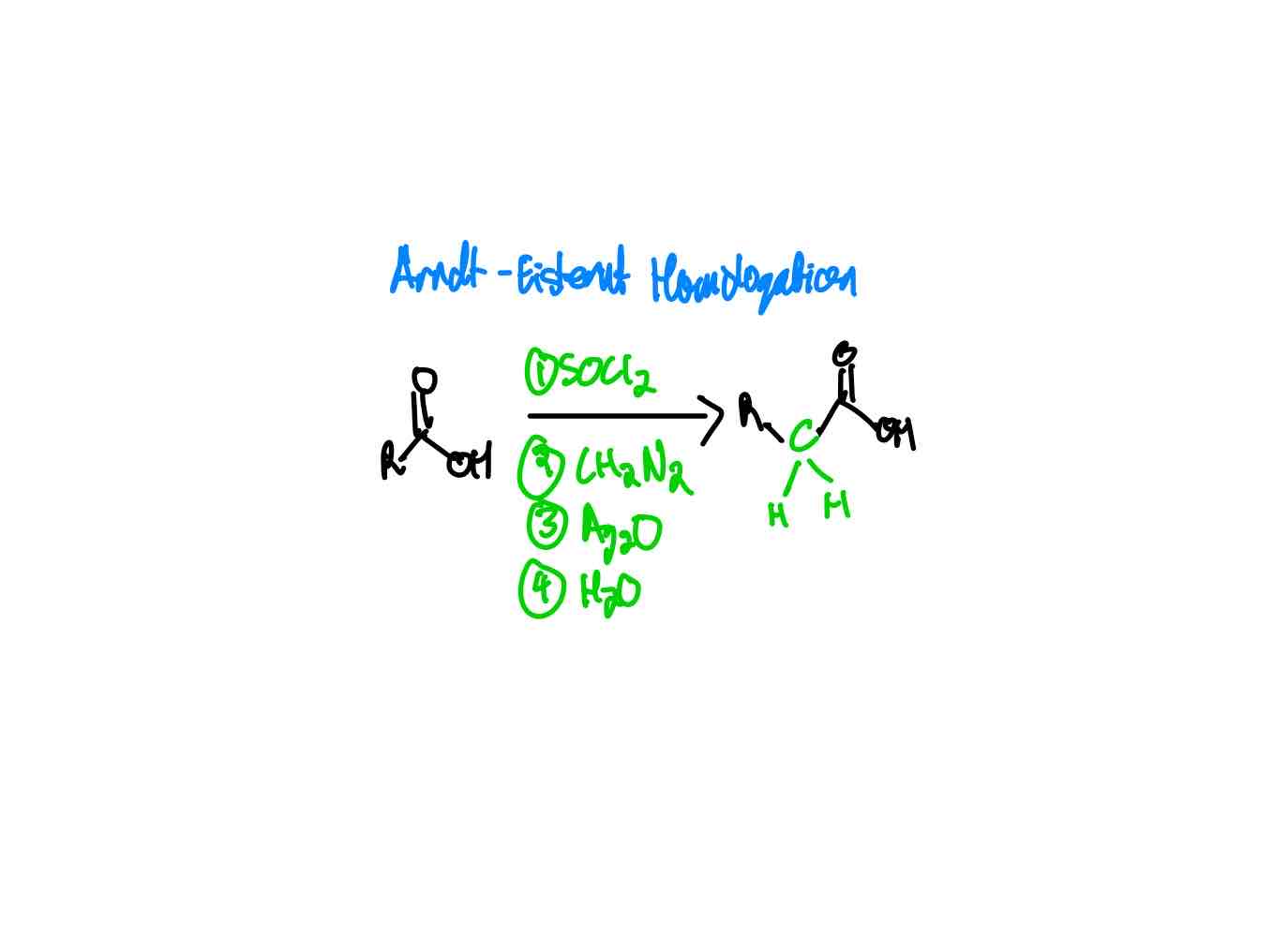
If you wanted to extend the R group of a carboxylic acid by one carbon, how would you proceed?