Molecular Shapes for VSEPR theory
1/12
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
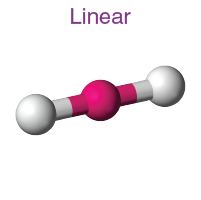
Linear
• Electron Domains: 2
• Lone Pairs: 0
• Bond Angle: 180°
• Polarity: Nonpolar (if atoms are identical)
• Example: CO₂
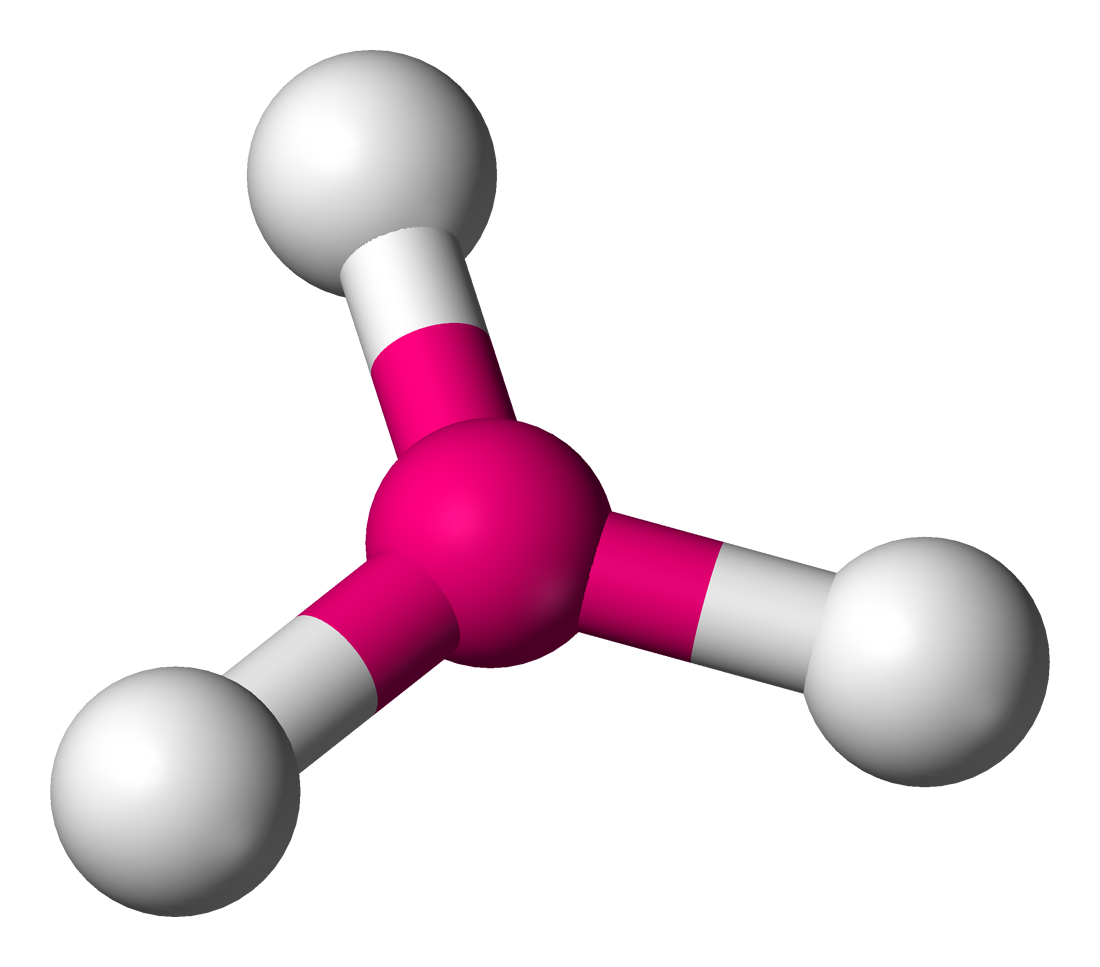
Trigonal Planar
• Electron Domains: 3
• Lone Pairs: 0
• Bond Angle: 120°
• Polarity: Nonpolar (if atoms are identical)
• Example: BF₃
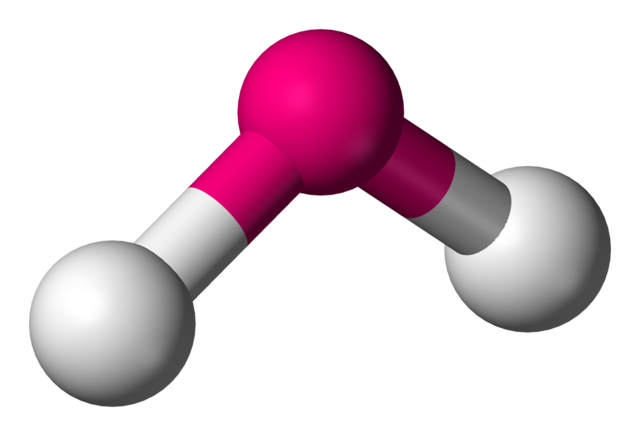
Bent (trigonal planar)
Electron Domains: 3
• Lone Pairs: 1
• Bond Angle: <120° (~117°)
• Polarity: Polar
• Example: SO₂
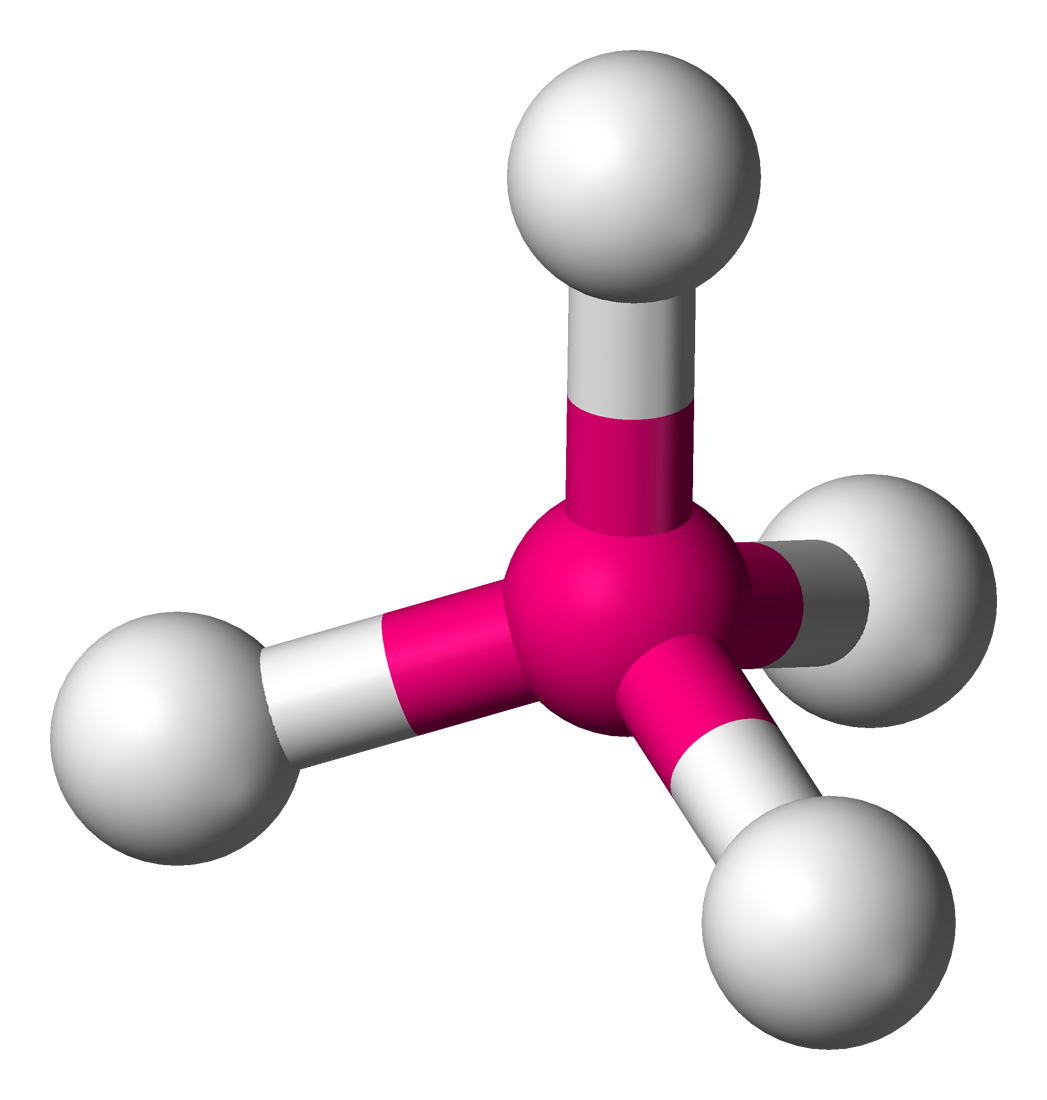
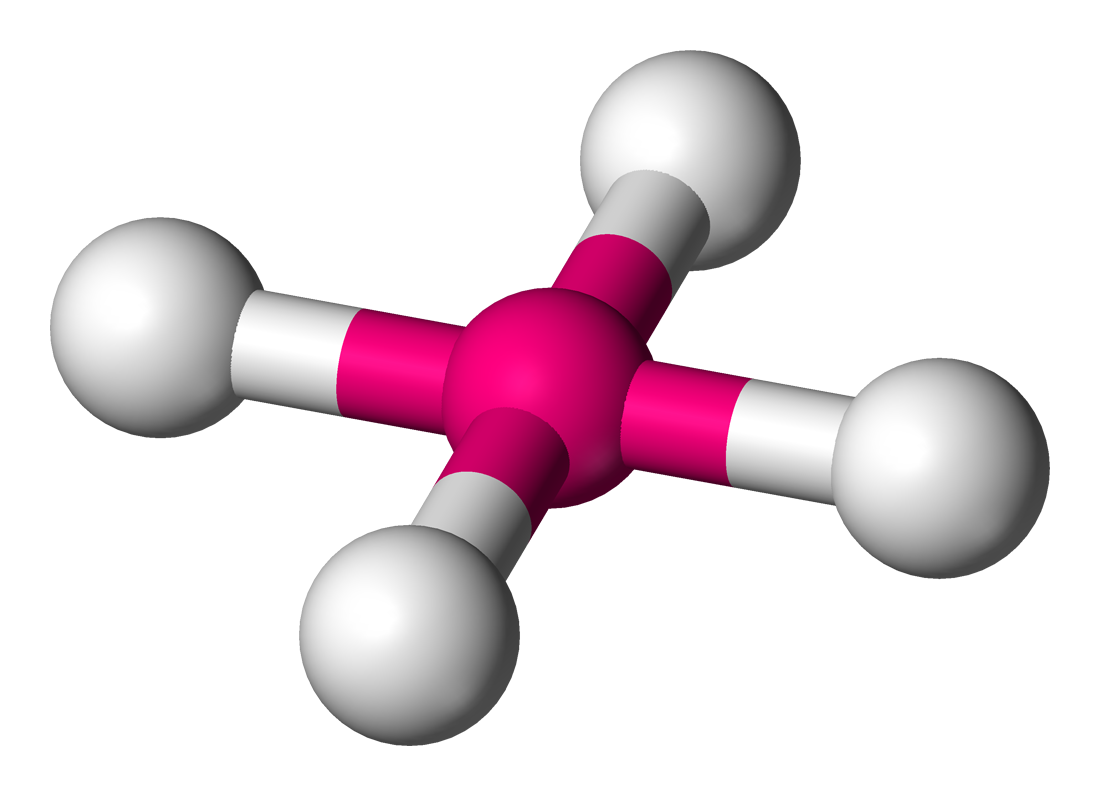
Tetrahedral
• Electron Domains: 4
• Lone Pairs: 0
• Bond Angle: 109.5°
• Polarity: Nonpolar (if atoms are identical)
• Example: CH₄
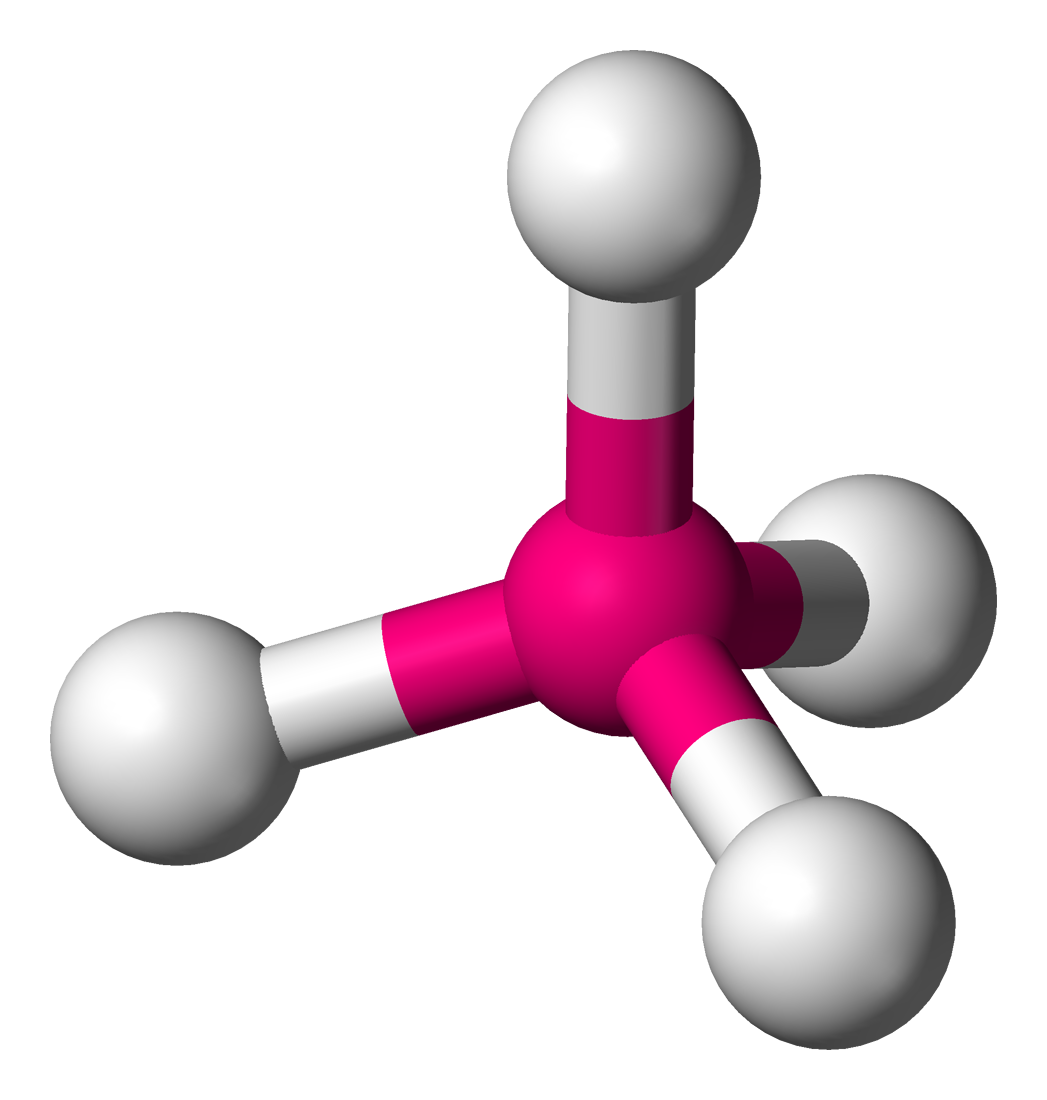
Trigonal pyramidal (tetrahedral)
• Electron Domains: 4
• Lone Pairs: 1
• Bond Angle: <109.5° (~107°)
• Polarity: Polar
• Example: NH₃
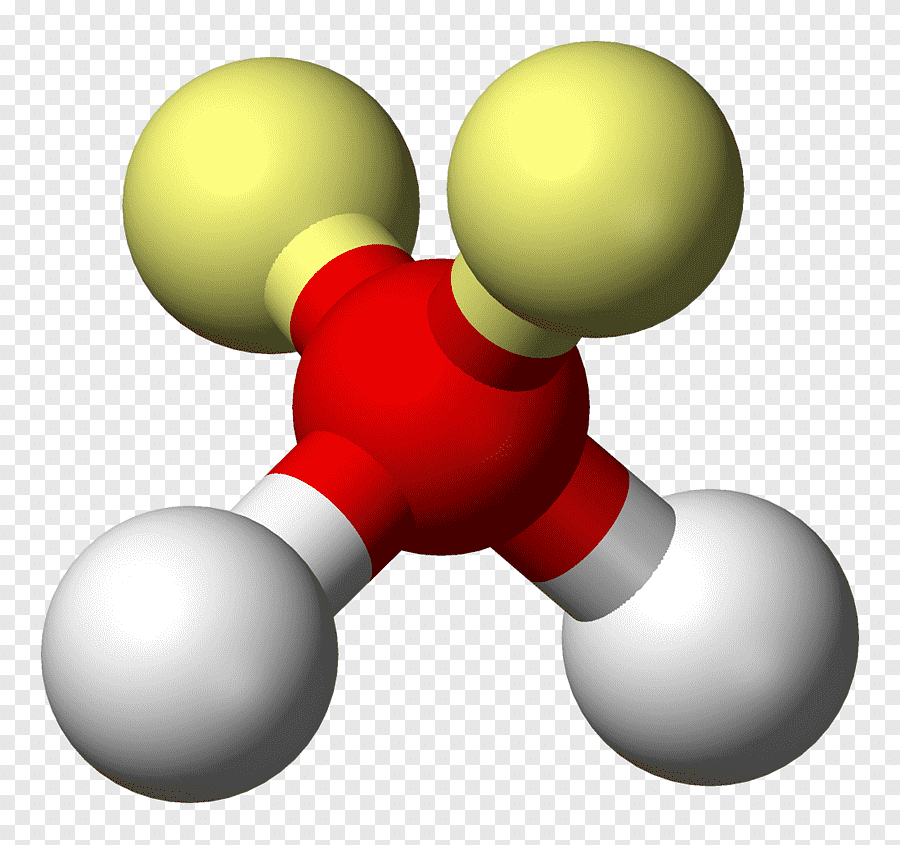
Bent (tetrahedral)
• Electron Domains: 4
• Lone Pairs: 2
• Bond Angle: <109.5° (~104.5°)
• Polarity: Polar
• Example: H₂O
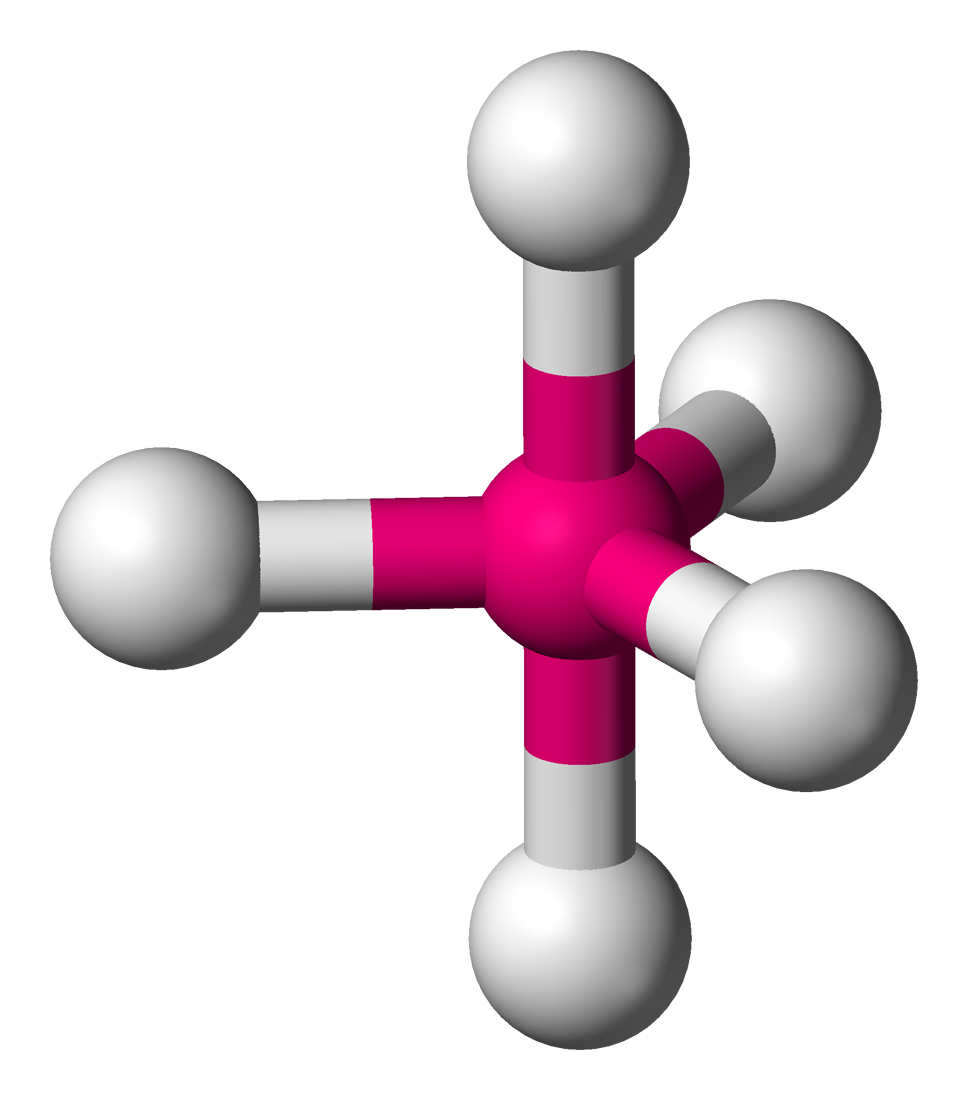
Trigonal bipyramidal
• Electron Domains: 5
• Lone Pairs: 0
• Bond Angle: 90° (axial), 120° (equatorial)
• Polarity: Nonpolar (if atoms are identical)
• Example: PCl₅
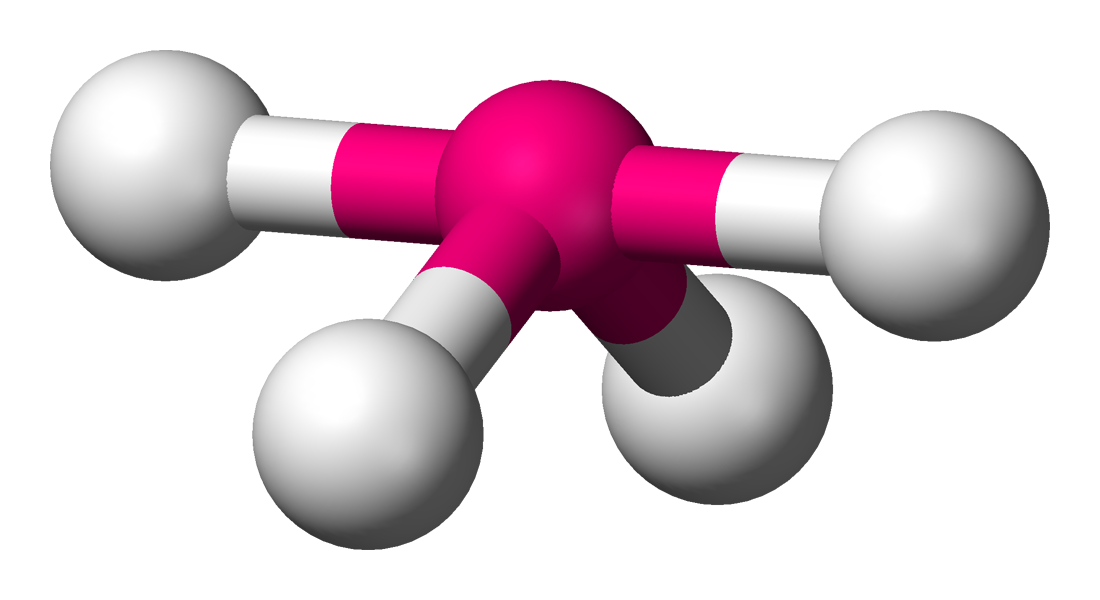
Seesaw
• Electron Domains: 5
• Lone Pairs: 1
• Bond Angle: <90° (axial), <120° (equatorial)
• Polarity: Polar
• Example: SF₄
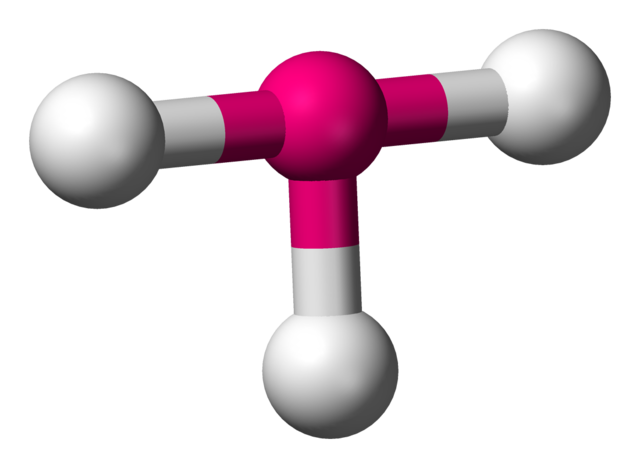
T-shaped
• Electron Domains: 5
• Lone Pairs: 2
• Bond Angle: <90°
• Polarity: Polar
• Example: ClF₃
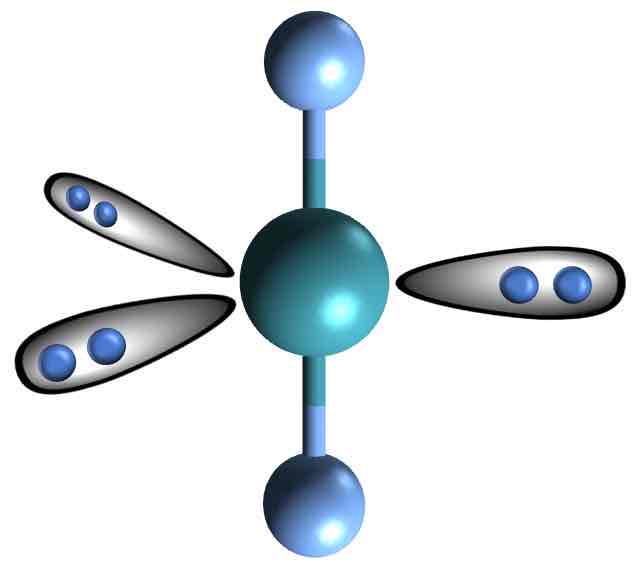
Linear (Trigonal bipyramidal)
• Electron Domains: 5
• Lone Pairs: 3
• Bond Angle: 180°
• Polarity: Nonpolar (if atoms are identical)
• Example: XeF₂
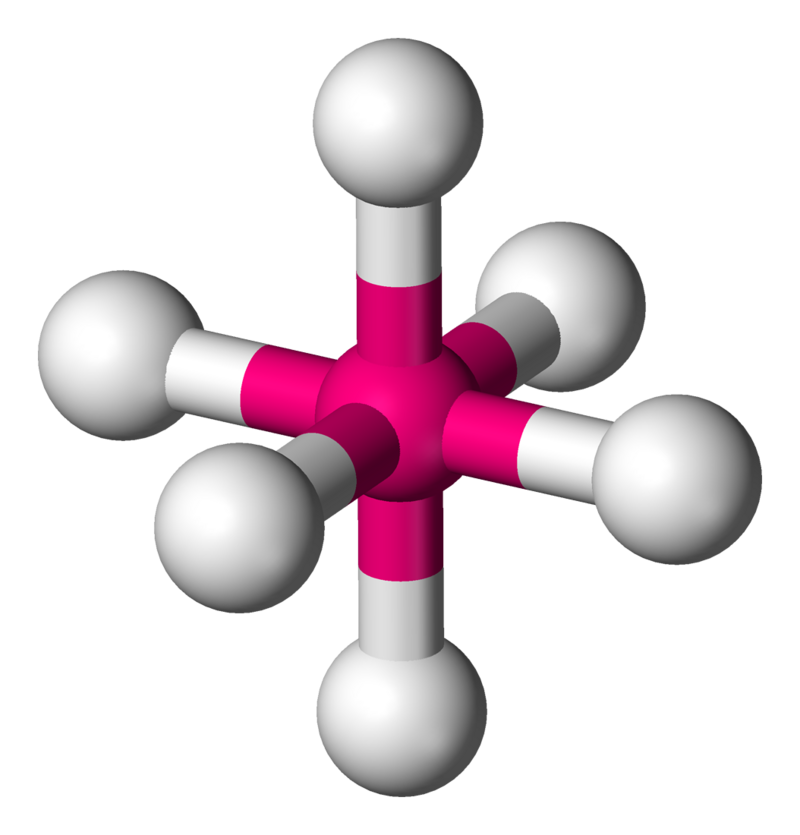
Octahedral
• Electron Domains: 6
• Lone Pairs: 0
• Bond Angle: 90°
• Polarity: Nonpolar (if atoms are identical)
• Example: SF₆
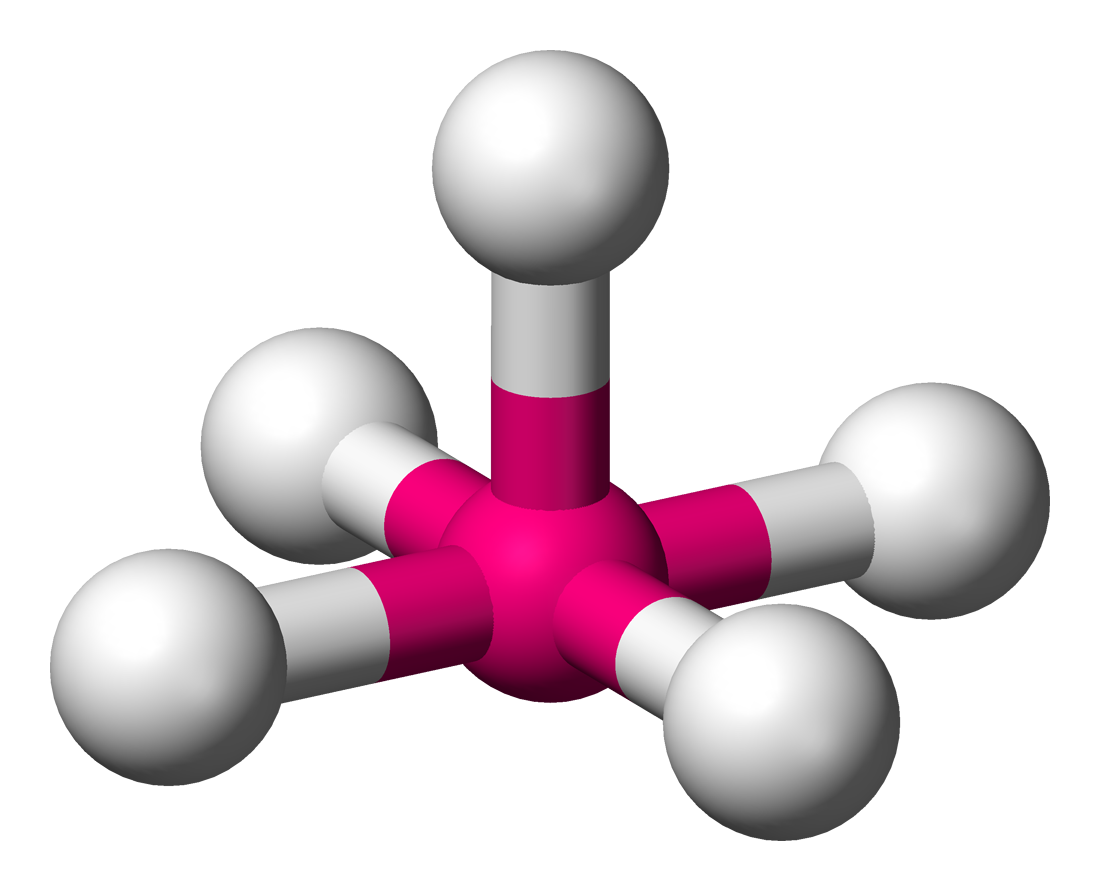
Square pyramidal (octahedral)
• Electron Domains: 6
• Lone Pairs: 1
• Bond Angle: <90°
• Polarity: Polar
• Example: BrF₅
Square planar (octahedral)
• Electron Domains: 6
• Lone Pairs: 2
• Bond Angle: 90°
• Polarity: Nonpolar (if atoms are identical)
• Example: XeF₄