Covalent bonding
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
molecule
uncharged group of two or more atoms held together
Covalent bonds are formed when
atoms share a pair of electrons
properties substances made of molecules…(2)
have low melting and boiling points
don’t conduct electricity
The length of a single covalent bond is related to the…(2)
size of the atoms
the distance between the nucleus and outer shell of electrons for each atom
The length of a double bond between two atoms is...
shorter than a single bond between the same atoms
longer than a triple bond between the same atoms
Predict which bond is longer.
C≡N
C≡C
Carbon is larger than nitrogen, so a C≡C bond should be longer than a C≡N bond.
Why is carbon larger than Nitrogen ?
Both nitrogen and carbon’s outer electrons are in the second shell.
Nitrogen has a larger nuclear charge, so its outer electrons are held closer (on average) to the nucleus.
The strength of a single covalent bond is related to…(2)
the length of the bond
the size of the atoms involved
trend in the strength of double, single and triple bond?
single<double<triple
provided between the same atom
Predict, based on bond lengths, which bond is stronger.
H-S
H-O
Oxygen atoms are smaller than sulfur atoms
This means an H-O bond should be shorter than an H-S bond.
And this means that an H-O bond should be stronger than an H-S bond
A dative bond forms when…
one atom contributes both electrons to the shared pair
In “bonds as sticks” diagrams we represent dative bonds as…
an arrow
Forming a dative covalent bond requires…
a dative bond donor
a dative bond acceptor
What is the correct representation for a dative bond from phosphorus to hydrogen?
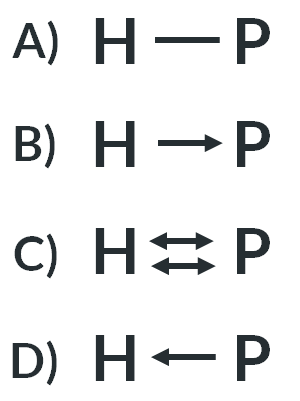
D