IB Chem Unit 7 (Gas and KMT)
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Kinetic Molecular Theory (KMT)
Gas particles are in constant, random, straight line motion
There are a lot of space between gas particles
Gas particles are so small, they occupy NO volume on their own.
If gas molecules collide, their energy remains constant (elastic collisions).
Gases do NOT attract one another. (They also don’t repel each other.
KMT and Temperature
Absolute temperature (measured in Kelvin) is directly proportional to particles’ kinetic energy
At the same temperature, all gases have the same energy.
At 0 K particles would be motionless
At 50 K particles have ½ the kinetic energy as at 100 K
0 K = -273 oC
Ideal vs. Real Gases: KMT Volume
Ideal gas particles are so small that the volume of the individual particles if they were at rest is essentially zero when compared to the total volume of the gas
Ideal Gas Particle
Have no volume
Real Gas Particles
Have volume
Ideal vs. Real Gases: KMT Motion
2. Ideal gas particles are in constant, rapid motion, moving in straight lines in all directions until they collide with other particles or the walls of their container.
Ideal Gas Particles
Move constantly in straight lines until collisions occur
Real Gas Particles
May move in curved paths
Ideal vs. Real Gases: KMT Collision
There are no attractive or repulsive forces between particles, and collisions between particles are elastic.
Ideal Gas Particles
No attraction (polarity), do not lose energy during collisions
Real Gas Particles
Have attractions (polarity), can lose energy during collisions
Ideal vs. Real Gases: KMT Condense
3. There are no attractive or repulsive forces between particles, and collisions between particles are elastic.
Ideal Gas Particles
Never condense
Real Gas Particles
As attractions increase, condensation occurs
What conditions will cause a gas to condense?
Forces of attraction
When gases are about to condense they become less like an ideal gas because:
Volume of the particles is more significant
Attractive forces between particles increase
Four Variables that Describe a Gas
Temperature
Volume
Pressure
Amount
Temperature
Average kinetic energy of gas particles
Must be in Kelvin
Absolute temperature scale
0 Kelvin = 0 mL = 0 Pa = 0 Energy
All atomic motion stops!
K = 273 + ºC
Note no degree symbol-absolute scale!
Volume
Length x width x height= m x m x m= m3
1 dm3 = 1 L
1 cm3 = 1 cc (cubic cm) = 1 mL
Volume can be in any unit but the units must cancel!
Pressure
Pressure is caused by collisions between gas particles and the container walls
Pressure Units
Force/unit area
101325 N/m2 =1 atm
100 kPa is STP pressure
Amount
Amount of gas in number of moles
Number of particles
STP
1 mol of any gas = 22.7 dm3
STP
Standard temperature and pressure
A standard set of conditions that we use to talk about gases
Must be easy to reproduce anywhere in the world
Temperature
0 oC
273 K (kelvin)
Pressure
100 kPa
Instruments
Manometer
Measures the pressure of an enclosed gas.
Barometer
Measures atmospheric pressure
A type of manometer
Diffusion of Gases
Diffusion describes how quickly gases mix with each other.
Gases tend to move from higher concentrations to lower concentrations.
Effusion of Gases
Effusion describes the movement of gases from an area of higher concentration into a vacuum
Gases with lower mass effuse more quickly than gases with higher mass.
KE = ½ mV2
Graham’s Law of Effusion
Effusion
The motion of gas through an opening into an evacuated chamber
The Gas with less mols move faster through a container.
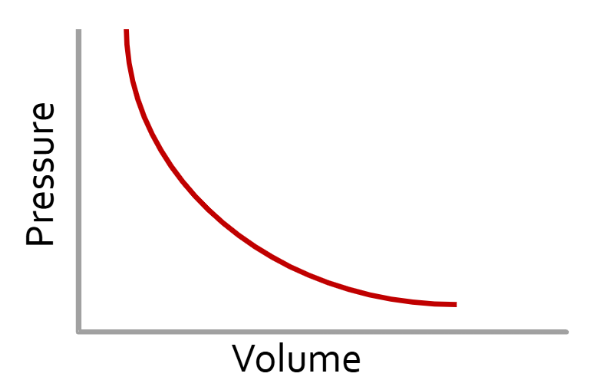
Boyle’s Law - Pressure and Volume
When the temperature is constant, the pressure and volume of a gas are inversely related.
As one goes up the other goes down.
P1V1 = P2V2
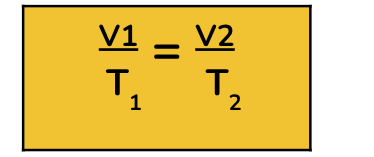
Charles’ Law - Volume and Temperature
When the pressure is constant, the volume and temperature of a gas are directly related.
As one increases the other increases.
Kelvin Conversion

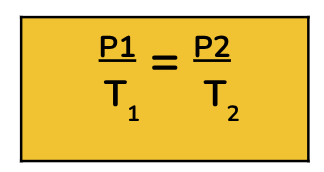
Gay - Lussac’s Law - Pressure and Temperature
When the volume is constant, the pressure and temperature (in kelvin) of a gas are directly related.
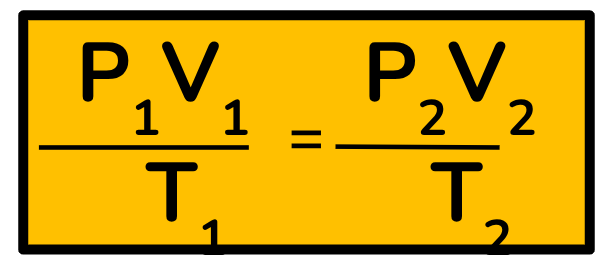
Combined Gas Law
Individual gas laws are combined
The number of molecules remains constant; everything else changes.
Pressure, volume & temperature may change.
This law allows us to figure out one variable when two of the others change.
Ideal Gases
To use the gas laws, we assume that gases behave ideally:
In reality, gases do not behave this way.
We make assumptions to make the math easier.
Characteristics of ideal gases:
Particles have no individual volume
No attractive forces between particles
Real gases behave this way ONLY at high temperatures and low pressures
Ideal Vs. Real Gases

Ideal Gas Law Equation
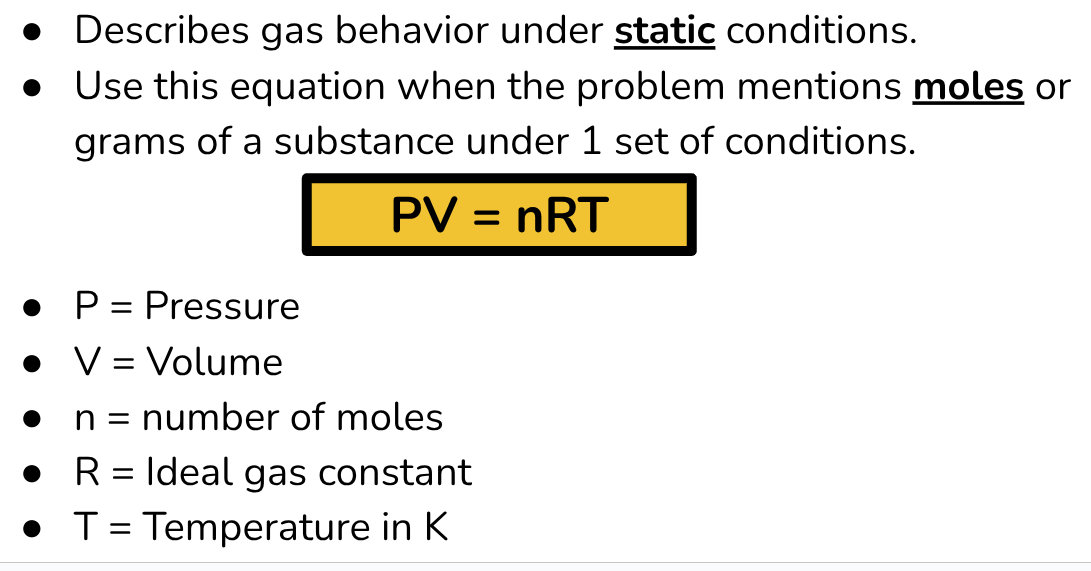
R value in Ideal Gas Law Equation
Avogadro’s Law
At constant temperature & pressure, given volumes of gas always contains the same number of particles
This means that the volumes of reactants/products are in the same ratio as the coefficients in the balanced equation
At STP
1 mol = 22.7 L
At room temperature
1 mol = 24.3 L