CME 304 Definitions
1/53
Earn XP
Description and Tags
Quiz 9/8
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
Thermodynamics
the study of energy transformations associated with material substances and of how these bodies are affected by the transport of different forms of energy into and out of them
Matter
all matter exists in the form of phases
solids, liquids, vapors, plasmas, supercritical fluids
a pure phase (atom/molecule) has a distinct molecular arrangement and chemistry
is homogeneous and isotropic throughout
is separated from other phases by a distinct boundary surface
cannot be a mizture of two or more phases of differing composition
exception = critical state of matter
phases of matter may exist in different forms
pure carbon exists as two solid phases (graphite/diamond)
He (two liquid phases, 3He and 4He)
Fe (three solid phases, one liquid phase, one vapor phase)
H2O (seven solid phases, one liquid phase, one vapor phase)
Solids
falls under “Intermolecular Forces in Pure Phases”
atoms or molecules arranged in a 3-dimensional network that is repeated in a fixed pattern (exception: glass)
attractive forces between atoms or molecules are strong enough to keep them in fixed equilibrium positions where there is a balance of attractive and repulsive forces
atoms or molecules oscillate (or vibrate) about their fixed equilibirum positions
magnitude of oscillations depends on temperature
when oscillations become too great, intermolecular forces are not strong enough to hold structure together and the —— tears apart and becomes a liquid
tldr: increased temperature increases oscillations, tearing apart the structure and changing its phase
liquids
falls under “Intermolecular Forces in Pure Phases”
intermolecular spacings are of the same order as in solids
atoms or molecules are no longer in fixed positions relative to each other
atoms or molecules can rotate and translate and vibrate more freely than in case of solids
intermolecular binding forces are weaker than in solids but still strong compared to gases
molecular volume is generally larger than solids
(exception: liquid water and ice)
tldr: less strong IMF but still some structure, can rotate, translate, and vibrate more than solids but less than gas
gases
falls under “Intermolecular Forces in Pure Phases”
molecules are far apart leading to lower densities and larger molecular volumes
particles move in random fashion
particles continually collide with each other and with the container walls
weak IMF (except at high pressures)
particles have high kinetic energy level compared to liquids and solids
molecules can rotate and vibrate within
must release a large amount of energy to condense into liquid or solid phases
tldr: molecules are far apart and free to move, continuous collisions with each other and container walls = increased/high kinetic energy
Energy (in thermodynamics)
a characteristic (or property) of a finite material body in equilibrium with its surroundings that gives it the capacity to convey some portion of this characteristic via thermal (heat) and/or mechanical (work) means to its immediate surroundings (or vice versa)
thermodynamics is the study of how the energy of such a material body can interact in prescribed ways with its surroundings (i.e., across a physical boundary) to transfer work and/or heat
a body can change its physical or chemical state due to such an interaction with its surroundings
heating of liquid water (change of temperature/pressure), melting of ice (change of phase), expansion/compression of a fluid (change of shape), combustion of chemical fuel (change of chemical species), dissolution of sugar in water (change of chemical composition)
Energy Storage
a fundamental principle is that a material body can store its energy in different forms
electronic
nuclear
kinetic
vibrational
rotational
magnetic
gravitational
surface
it is assumed that any interaction of a material body with its surroundings could result in the changes in the relative amounts of energy storage
Energy Transformations
fundamental principle of thermodynamics
Different forms of energy can be transformed or exchanged with each other
i.e.
electrical to thermal (light bulb)
mechanical to electric (power station)
chemical to thermal (combustion of a fuel)
thermal to mechanical (auto piston)
nuclear to thermal (nuclear reactor)
Property
coordinate
a macroscopic characteristic of a finite body of matter that describes the internal state of the system
a specific numerical value can be assigned without knowledge or reference to its previous history
it is time independent
uniform throughout (homogeneous)
independent of direction of measurement (isotropic)
independent of path chosen
typical properties might be:
pressure
volume
temperature
tldr: often the thing we are solving for or using to solve for something else, it describes the internal state of the system and can be a numerical value
Absolute Property
does NOT depend on a choice of reference state
Density
Heat Capacity
Thermal Expansion
Compressibility
Electrical conductivity
Floating property
properties that cannot be measured directly but must be computed relative to some arbitrarily assigned reference condition
there are no meters or devices for measuring ———- ————
only property changes can be determined
property value depends on choice of reference state
if we arbitrarily set U (internal energy) = 0 at T = 0 degrees C and P = 1 bar, then all calaculations of internal energy are made relative to this reference condition
since we are generally more interested in determining property changes than in absolute property values, the choice of reference condition does not matter since it cancels out
tldr: the property we calculate the change of, not the actual value, need reference conditions in order to do this
examples:
internal energy
enthalpy
entropy
Gidds Free Energy
Gravitational potential energy
Chemical potential
Kinetic energy
*most thermodynamic properties are ——— ——— in they “float” depending on choice of reference state
Extensive property
DEPEND on the SIZE of the system
if a system is sub-divided into smaller units and a system property is identified then the extensive value of that property is the sum of all the sub-units that make up the system (i.e. volume, area, mass)
extensive properties can change with time as a system interacts with its surroundings
tldr: key word DEPEND
Intensive property
INDEPENDENT of the size of the system and may vary from place to place within the system at any moment of time
can vary with both position and time as a system interacts with its surroundings
ex: pressure, temeprature, heat capacity, density, specific or molar volume, volume expansion coefficient, compressibility, chemical composition, specific internal energy or enthalpy
tldr: key word INDEPENDENT
Non-property
a quantity (or characteristic) is a ——— ———- if its change in value between two states depends on the details of the process and not solely on the end states themselves
heat transfer between two bodies at different temperatures
mechanical energy transfer between two different bodies (work)
tldr: its value depends on the process more than the end states of it, unlike a property, it is not independent of the details of the process
Thermodynamic state of a body
the sum totality of all its properties
since there are often known mathematical relations between different properties, the thermodynamic state can often be described as a subset of properties from which other properties can be determined
thermodymic properties are uniform throughout body
thermodynamic properties are time independent
control properties
properties which can be externally prescribed to alter the energy state of a materials system
pressure
temperature
volume
chemical composition
dependent properties
properties that are determined (or dependent on) the setting of controllable properties
internal energy, enthalpy, entropy, free energy
heat capacity (at cont T or V)
volume thermal expansion
compressibility
macrostate
any state where the thermodynamic properties can be easily measured using laboratory equipment (i.e. P,T, V)
we will be conerned here only with the thermodynamic behavior macroscopic systems since the study of microscopic is beyond the current scope of this discussion
microstate
any state where the thermodynamic properties are determined by microscopic quantum mechanical parameters
this is the realm of statistical thermodynamics and is beyond the scope of this course
equtions of state
it has been shown that a mathematical relationship exists among the thermodynamic properties (P,V,T) that specify the equilibrium thermodynamic state of the system
for an ideal gas we need 3 properties (i.e. P,V,T)
PV = nRT
VanderWaals gas
(P+a/V2)(V-b)=NkT
thus if we specify any two thermodynamic properties, the ———- — —— will, in principle, determine the third
thus the thermodynamic state of a system can always be specified by any two thermodynamic properties and the ——— — —— yields the 3rd
tldr: essentially the principle that if you are given two properties, this can be used to determine the third, think physics equations
thermodynamic system
a system is any object, or finite quantity of matter that occupies a region of space that is selected to be set aside for study
the system is treated as a whole unit
the system can be described by a set of properties that apply to the system as a whole
system properties can be changed by interaction with surroundings
system may exchange energy or material flow with surroundings
the # of system properties used to describe the system is small (i.e. 3-6)
*make sure you KNOW THIS
sub-system
a ———- is obtained when a thermodynamic system is divided into two or more sub-parts separated by a sub-system boundary and each subsystem treated as a separate entity as shown below
any property changes in the system is the result of summing the property changed in each sub-system that make up the system
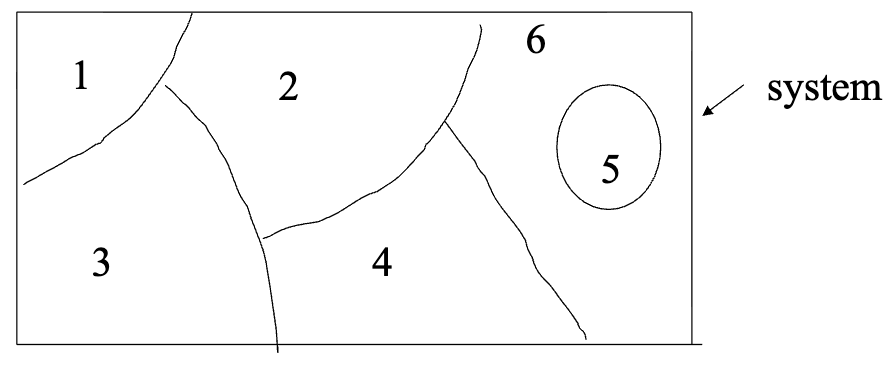
macroscopic system
a system that is treated as a whole unit
the paramters that describe the system must apply to system as a whole (i.e. P, T, V, X=composition)
the number of thermodynamic coordinates used to describe system is small (i.e. 3-6)
microscopic system
a system treated as a collection of minute atomic/molecular discrete entities
system varibles apply only to individual particles
number of coordinates need to describe system is very large (1023)
relation of microscopic to macroscopic system properties is treated in statistical thermodynamics
surroundings
the portion that is NOT the system
only includes that portion of the immediate space in the neighborhood of the system that is capable of interacting with the system
can absorb heat from or release heat to the system through boundary
can have work performed on it by the system or can do work on the system
can absord matter from the sysbtem or release matter into the system
*KNOW THIS
system boundary
we assume that a distinct physical and/or chemical ——— (or interface) forms at the periphery of the system and separates the system from its surroundings
may or may not allow the system to interact with its surroundings
if it permits heat transfer and mechanical energy transfer into or out of the system but NOT mass flow, it is CLOSED
if it does NOT permit heat transfer into or out of the system, it is an adiabatic boundary
if it permits heat transfer, mechanical energy transfer and mass flow into or out of the system it is OPEN
if it is fixed then it is a rigid ———- (rigid container): otherwise if the ———- can be displaced it is a moveable ———— (piston; cylinder)
if an open system has particular locations where it can interact with its surroundings, it is either an entrance or exit port ocated at the —————
*KNOW THIS
universe
includes both the system + surroundings
_________ = system + surroundings
any changes (i.e. energy, entropy) in the _______ must be the result of the combined changes in the System and Surroundings
thus, we may write:
delta(______) = delta(System) + delta(Surroundings)
*KNOW THIS
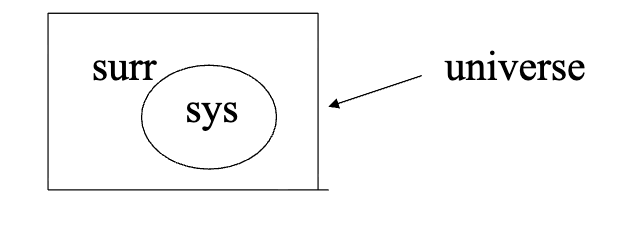
types of thermodynamic systems
there are three types of systems that we will consider:
closed system
isolated system
open system (control volume)
see further cards for definitions
closed system
system which is in thermal or mechanical contact with its surroundings but does NOT allow the passage of matter into or out of it
heat and/or work can be performed on the system by surroundings or vice versa
isolated system
system is NOT in thermal, mechanical, or physical contact with the surroundings
e.g. a thermos bottle
open system
aka control volume system
system not only is in thermal and mechanical contact with its surroundings but also will allow the passage of matter through it
*think of a nozzle
thermodynamic equilibrium
thermodynamics is concerned with equilibirum states of matter
implies a state of matter in which all the forces acting on a system are in balance
there are four basic types of equilibrium that are of interest
mechanial equilibrium
thermal equilibrium
phase equilibrium
chemical equilibrium
criteria:
isolate system from surroundings
look for measureable or observable changes in system properties
if no changes occur over time, the system is in eq at the time of isolation and is said to be in its equilibrium state
there is NO requirement that system be in an equilibrium state during changes of state
mechanical (hydrostatic) equilibrium
no unbalanced forces acting on the system
system is stationary in time and space
thermal equilibrium
no temperature gradients throughout system
system must be at uniform temperature
no net heat flow between different systems
chemical equilibrium
no chemical reactions take place at measurable rates
rate of forward reactions = rate of reverse reactions
phase equilibrium
two or more different phases of matter (solid, liquid, vapor) co-exist at a given P and T
driving forces for equilibrium
changes of state occur bc of the presence of an imbalance in driving forces that cause the system to move from one equilibrium state to another
these driving forces (or constraints on the system) are the result of:
temperature differences leading to flow of heat between systems and surroundings
pressure differences leading to expansion or contraction of system/surroundings
compositional differences leading to the diffusion chemical species between regions of differing composition
chemical reactions between different reacting species to produce new chemical species
temperature, pressure and/or compositional difference that lead to the formation of a new phase
exception: the presence of energy barriers can prevent equilibrium from occurring in some systems
we conclude that the system is at equilibrium when all driving forces are uniform throughout system and surroundings and that all barriers to change have been overcome
equilibrium postulates
all thermodynamic systems will spontaneously tend to move toward their equilibrium states
the equilibrium state will be determined by the constraints imposed on the system (P,T,V,X) where X=composition
the presence of thermodynamic energy barriers may prevent equilibrium from being attained
leads to metastable equilibrium states
Classical Thermodynamics is based upon the determination of the equilibrium states of matter
all changes of state are assumed to be from one equilibrium state to another equilibrium state
once the equilibrium state has been achieved, the prior history of the system is of no relevance
thermodynamic process
takes place when a system is forced to leave its equilibrium state due to interaction with the surroundings and move toward another equilibrium state dictated by the new set of constraints imposed on it
any such changes of equilibrium condition is referred to as a change of state
two different states must be distinguishable by having measurably different properties
all thermodynamic properties are independent of the choice of path
thermodynamics is the study of processes leading to a change in thermodynamic properties of a system
path
a specific pre-selected process whereby a system changes its properties according to a prescribed set of parameters that describe the path
a system can change its state via different paths
a system can move along an open path or a closed path
an open path does not allow the system or return to its original starting point (state)
a closed path allows the system to return to its original starting point
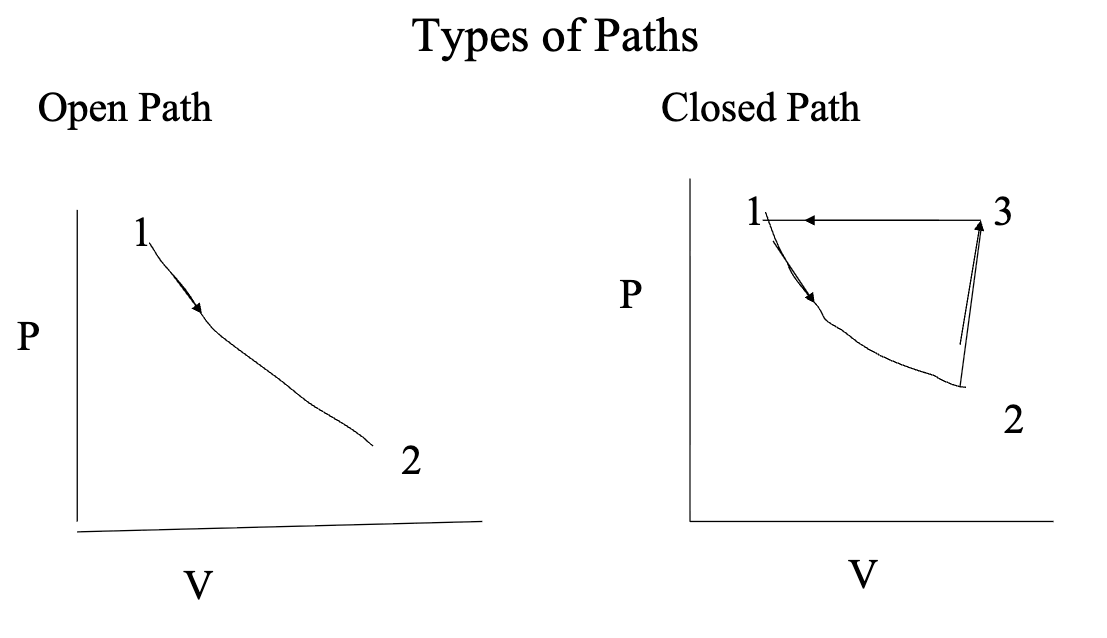
constraint
a constraint on a thermodynamics process occurs when a thermodynamic property is fixed at a pre-selected value and the thermodynamic process is carried out with this fixed value throughout
most thermodynamic processes are carried out with at least one constraint:
const. temp
const. pressure
const. volume
const. chemical composition
no heat flow
no mass flow
infinitesimal process
a process carried out such that only infinitesimal changes in thermodynamic coordinates can occur (i.e. dT, dV, dP)
finite process
takes place where finite changes in thermodynamic coordinates can be measured (deltaT, deltaP, deltaV)
quasi-static process
a process carried out so slowly that the system is always close to thermal, mechanical, or chemical equilibrium
rate of change of process must be much slower than the time it takes for system to respond to external changes (e.g., slow withdrawal of a frictionless piston inside of piston-cylinder assembly containing a gas)
isothermal
type of thermodynamic process
process carried out such that the system temperature remains constant throughout
adiabatic
type of thermodynamic process
process carried out such that no heat is allowed to enter or escape the system (isolated)
isobaric
type of thermodynamic process
process carried out such that the system pressure remains constant throughout
isometric
type of thermodynamic process
process carried out at constant volume
reversible process
a process in which a system and all parts of the surroundings can be restored to their initial states after a given process is reversed along the same path
________ processes are idealized processes that do not occur in nature
________ processes are defined to allow making important thermodynamic calculations and comparisons
delta(Universe) = delta(System) + delta(Surroundings)=0 or
delta(System) = -delta(Surroundings)
can be thought of as the limiting case in which irreversibilities within the system or surroundings are reduced to their lowest values
all attempts to reduce spontaneous changes within the system will increase reversibility
irreversible process
a process in which the system or surroundings. (or both) are permanently changed when the process is reversed from the final to initial state
they occur in most naturally occuring changes
difficult the quantiy accurately
delta(Universe) = delta(System) + delta(Surroundings) =/ 0
examples
unrestrained heat transfer between heat conducting bodies with finite temperature differences
unrestrained expanson of a gas at high pressure to a lower pressure
spontaneous chemical reaction (combustion)
spontaneous mixing of materials of two different compositions
friction due to rubbing of surfaces
electric current flow through a resistance
a chemical explosion
cracking of an egg
erosion of a mountain by wind and rain
summary of reversible processes
processes that are carried out in which no barrier to system movement from one equilibrium state to another are present
system is never more than differentially removed from equilibrium state
process is quasi-static
process traverses through a succession of equilibirum states
driving forces are differential in magnitude
process can be reversed by reversing direction of differential driving forces
initial system state is restored after reverse process is completed
internal irreversibilities
those irreversible processes that occur within the system boundary
external irreversibilities
those processes that occur outside the system boundary (i.e. surroundings)
equilibrium and reversibility postulate
all processes that deviate differentially from their equilibrium state are considered to be reversible
processes that deviate differentially from their equilibrium state are assumed to be so close to equilibrium that the likelihood of irreversibilities creeping in is thought to be very small
if system energy at equilibrium is U0, then a disturbance in, say, temperature of +dT might bring the system into a new state, U0+dU, such that the system can be returned infinitesimally and reversibly to its initial state by simply reversing the direction of temperature, -dT