binary diagrams
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
do you add 1 or 2 at the end of the phase rule for binary systems?
+1
how do you find weight % of a mineral?
EX: if you combine 1 gram of NaAlSi3O8 with 8 grams of CaAl2Si2O8 to form a single grain of plagioclase, what is the weight fraction of Ab and An in the resulting plagioclase?
NaAlSi3O8 = Albite…. 1gAb/9gtotal = 11% (Ab11)
CaAl2Si2O8 = Anorthite… 8gAn/9gtotal = 89% (An89)
how many components (for the phase rule) do binary diagrams have?
always 2!
how to determine phases (for phase rule) in binary diagrams
phases change based on whether you have a solid, liquid, both… or you’re at the eutectic (p = 3)
what are the intensive and extensive variables for the Ab, An binary diagram with a solid solution?
intensive = temperature, composition
extensive = weight of An and Ab
why does the Ab - An binary eutectic diagram have a solid solution?
because intermediate plagioclase compositions exist
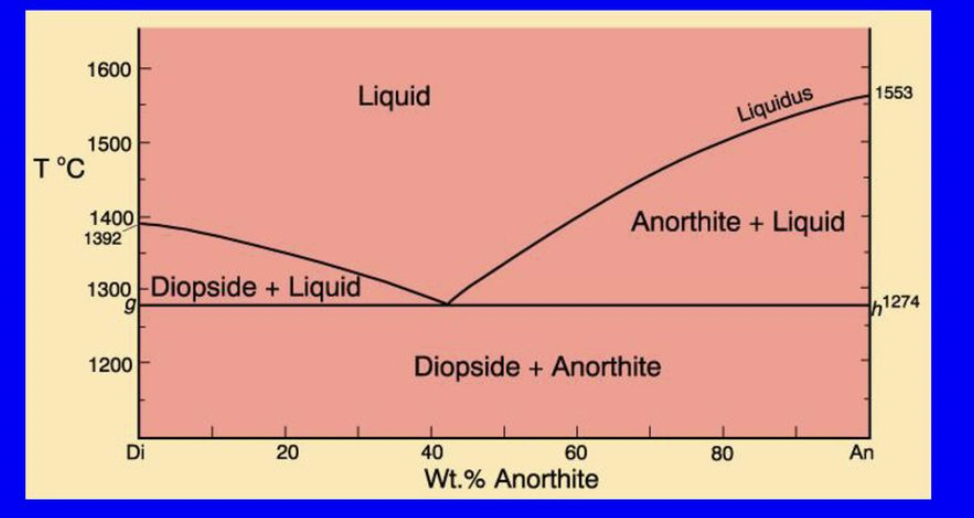
what happens at the eutectic point in the Di-An binary eutectic?
Di begins crystallizing, and a discontinuous rxn occurs where the T is stable until all the liquid melt is crystallized
what is the composition of the first liquid melt when melting? the last liquid melt when crystallizing?
composition of first and last liquid melt is always at the eutectic point
what rock type from the stillwater complex lab could be explained w the Di-An binary eutectic diagram?
20KNST-6
we called it an olivine-bearing gabbro but it could also have been called an anorthite based on this diagram
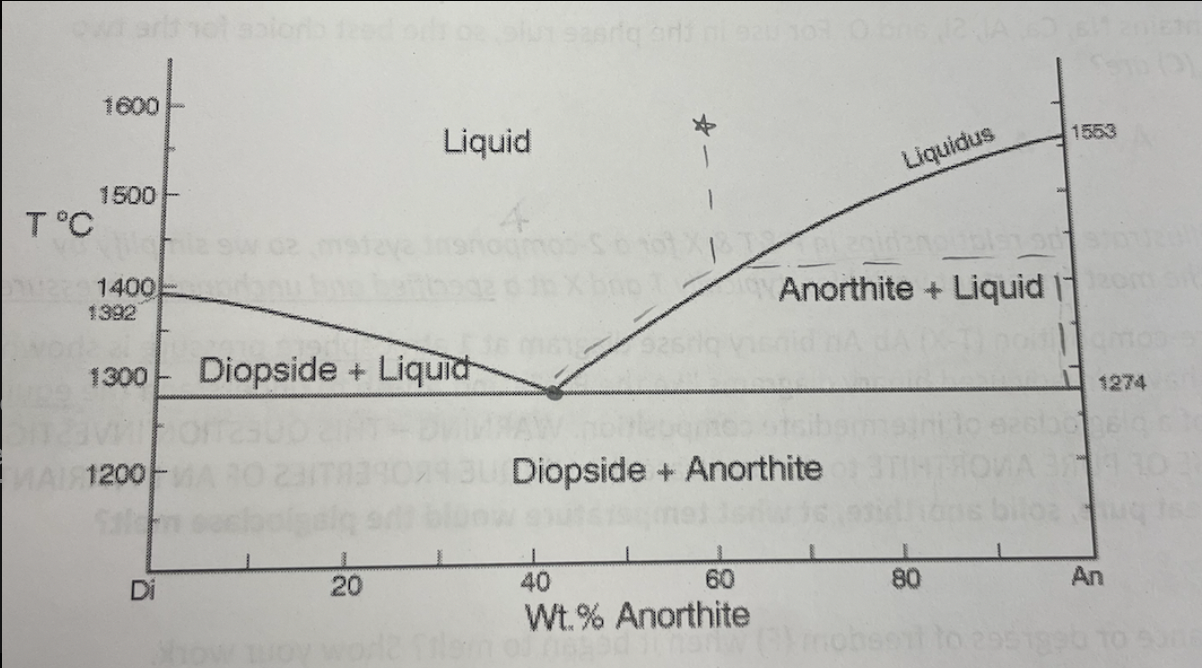
what temperature does pure anorthite begin crystallizing on the Di-An binary eutectic diagram?
at 1553! it hits the liquidus and begins equilibrium crystallization along the solidus (y-axis)
at what temperature does the eutectic occur on the Di-An binary eutectic
1274 degrees
what is a tie-line (on the Di-An binary eutectic)
the line that connects the point on the liquidus to the equilibrium point on the solidus
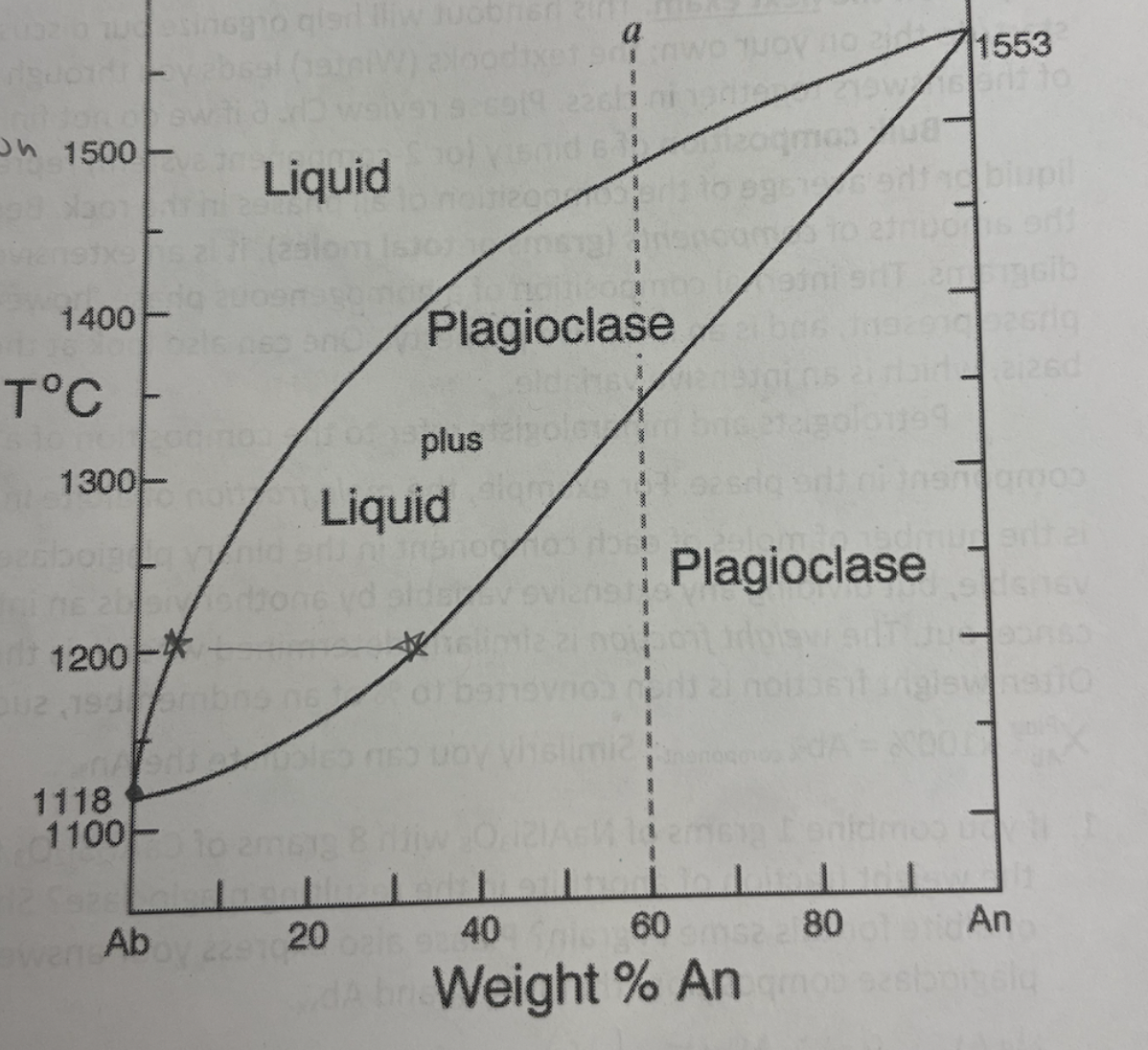
when is first solid produced on the Ab-An binary eutectic (with solid solution)
at around 1500 when melt hits liquidus (and therefore solidus)
what is the phase rule at the eutectic (1274)
F=2-3+1=0
0 degrees of freedom! every component is fixed
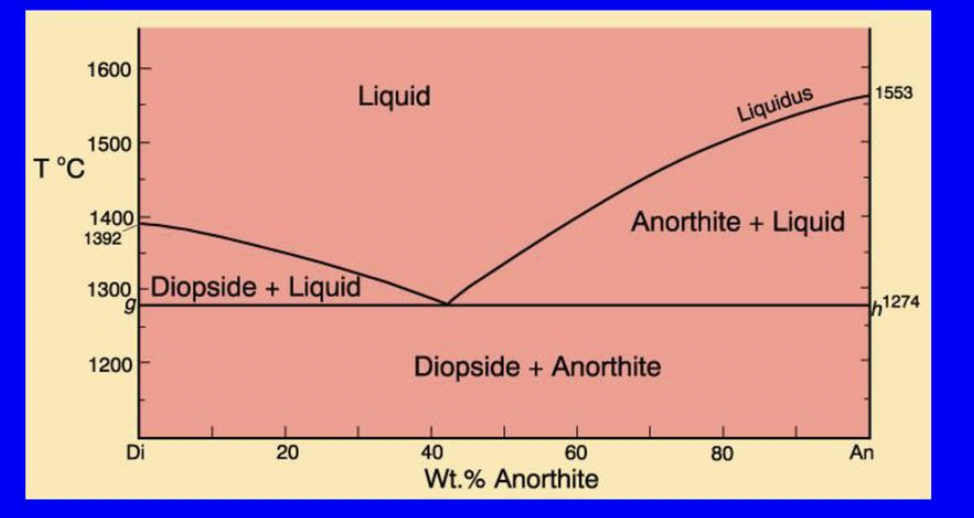
at what temperature will F first change in the Di-An binary eutectic diagram?
at around 1553 when the melt reaches the liquidus and phase changes from 1 phase (liquid) to 2 phases (liquid and solid)
what type of rxn occurs along the liquidus on the Di-An from the first appearance of a solid?
continuous rxn - liq crystallizing into solid An over a range of T
what must happen before this isobaric can reach a lower temperature?
all liquid must be crystallized so T can drop once a solid rock has formed
why do most basalts erupt at temperatures of 1250 or lower
they have to erupt before they reach the eutectic point (point of last liquid appearance) where almost all melt is crystallized or else it would be a solid eruption
what does the melt consist of if it’s located below An40?
pure Di melt! solid will always have 50/50 Di and An in it because they are both crystallized at the eutectic before they fully solidify
what is variance at all eutectic points!
0