Addition Reactions
1/69
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms
Hydrohalogenation of Alkene Transformation
Alkene to alkyl halide
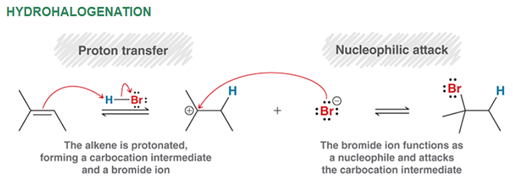
Hydrohalogenation of Alkene (non ROOR) Regiochemistry and Stereochemistry
R: Markovnikov
S: Racemic
Hydrohalogenation of Alkene (ROOR) Regiochemistry and Stereochemistry
R: Anti-Markovnikov
S: Racemic
Hydrohalogenation of Alkene Reactants
Alkene + HBr in CCl4 or H2O2
Acid-Catalyzed Hydration of Alkene Transformation
Alkene to alcohol
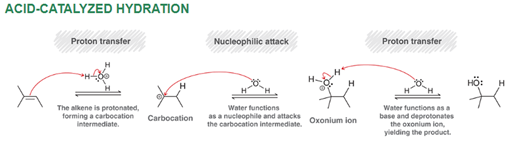
Acid-Catalyzed Hydration of Alkene Regiochemistry and Stereochemistry
R: Markovnikov (possible rearrangement)
S: Racemic
Acid-Catalyzed Hydration of Alkene Reactants
Alkene + acid + H2O or ROH
Oxymercuration-Demercuration of Alkene Transformation
Alkene to alcohol
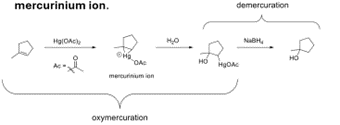
Oxymercuration-Demercuration of Alkene Regiochemistry and Stereochemistry
R: Markovnikov
S: Racemic
Oxymercuration-Demercuration of Alkene Reactants
Step 1: Alkene + Hg(OAc)2 + nucleophile, H2O, or ROH
Step 2: Mercurinium ion + NaBH4
Hydroboration-Oxidation of Alkene Transformation
Alkene to alcohol
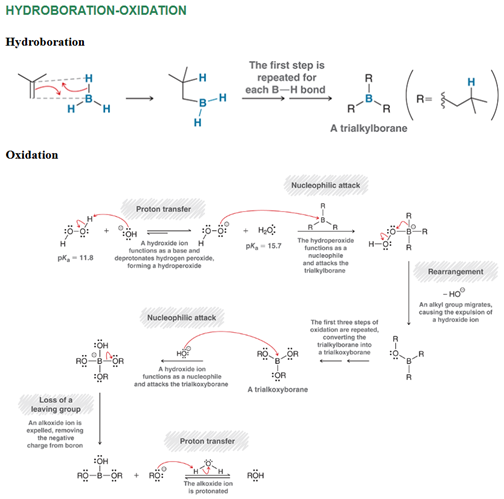
Hydroboration-Oxidation of Alkene Regiochemistry and Stereochemistry
R: Anti-Markovnikov
S: Syn addition
Hydroboration-Oxidation of Alkene Reactants
Step 1: Alkene + BH3 in THF
Step 2: H2O2 + NaOH
Catalytic Hydrogenation of Alkene Transformation
Alkene to Alkane
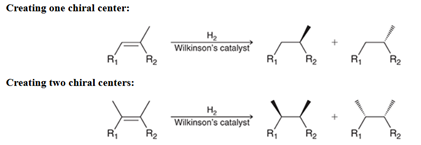
Catalytic Hydrogenation of Alkene Regiochemistry and Stereochemistry
R: Both sides add H
S: Racemic syn addition
Catalytic Hydrogenation of Alkene Reactants
Alkene + H2 + metal (Pt or Pd)
Halogenation of Alkene Transformation
Alkene to alkyl dihalide
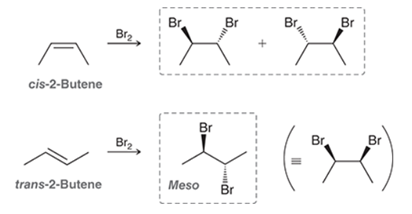
Halogenation of Alkene Regiochemistry and Stereochemistry
R: Vinyl
S: Syn for trans, anti for cis
Halogenation of Alkene Reactants
Alkene + Br2 or Cl2
Halohydrin Formation of Alkene Transformation
Alkene to alkyl halide with an alcohol
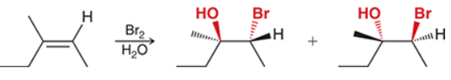
Halohydrin Formation of Alkene Regiochemistry and Stereochemistry
R: Alcohol Markovnikov, halide anti-Markovnikov
S: Anti
Halohydrin Formation of Alkene Reactants
Alkene + Br2 + xs H2O or other nucleophile
Anti Dihydroxylation of Alkene Transformation
Alkene to alkane with two alcohol groups
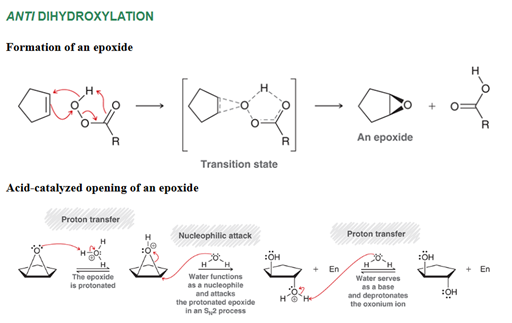
Anti Dihydroxylation of Alkene Regiochemistry and Stereochemistry
R: OH adds to both sides of pi bond
S: Anti
Anti Dihydroxylation of Alkene Reactants
Step 1: Alkene + RCO3H (peroxyacid) or peroxyacetic acid or MCPBA and H
Step 2: H3O+ or other acid
Syn Dihydroxylation of Alkene Transformation
Alkene to alkane with two alcohol groups
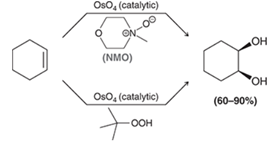
Syn Dihydroxylation of Alkene Regiochemistry and Stereochemistry
R: OH adds to both sides
S: Syn
Syn Dihydroxylation of Alkene Reactants
Step 1: Alkene + OsO4
Step 2: H2O + NaHSO3 or NMO
Oxidative Cleavage of Alkene Transformation
Alkene to mix of ketone and aldehyde (alkene can be cyclic)
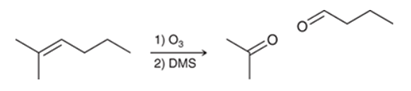
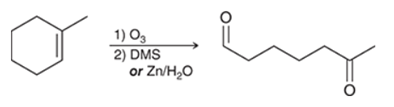
Oxidative Cleavage of Alkene Regiochemistry and Stereochemistry
R: N/A
S: N/A
Oxidative Cleavage of Alkene Reactants
Step 1: Alkene + O3
Step 2: DMS or Zn/H2O
Catalytic Hydrogenation of Alkyne Transformation
Alkyne to alkane
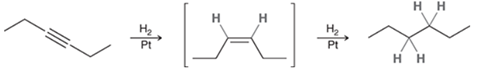
Catalytic Hydrogenation of Alkyne Regiochemistry and Stereochemistry
R: N/A
S: N/A
Catalytic Hydrogenation of Alkyne Reactants
Step 1&2: Alkyne + xs H2 + Pt or Pd
Poisoned Catalytic Hydrogenation of Alkyne Transformation
Alkyne to cis alkene

Poisoned Catalytic Hydrogenation of Alkyne Regiochemistry and Stereochemistry
R: N/A
S: Syn
Poisoned Catalytic Hydrogenation of Alkyne Reactants
Alkyne + 1 eq. H2 + poisoned catalyst (lindler’s catalyst or Ni2B)
Dissolving Metal Reduction of Internal Alkyne Transformation
Alkyne to trans alkene
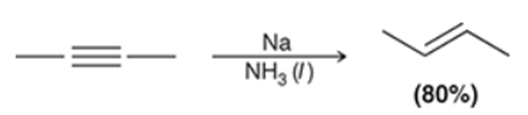
Dissolving Metal Reduction of Internal Alkyne Regiochemistry and Stereochemistry
R: N/A
S: Anti
Dissolving Metal Reduction of Internal Alkyne Reactants
Na in cold NH3
Hydrohalogenation of Alkyne Transformation
Alkyne to alkyl halide on alkene
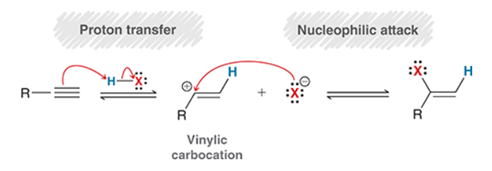
Hydrohalogenation of Alkyne Regiochemistry and Stereochemistry
R: Markovnikov
S: Racemic
Hydrohalogenation of Alkyne Reactants
Alkyne + 1 eq HX
Dihalide Hydrohalogenation of Alkyne Transformation
Alkyne to geminal dihalide on alkane

Dihalide Hydrohalogenation of Alkyne Regiochemistry and Stereochemistry
R: Markovnikov
S: Racemic
Dihalide Hydrohalogenation of Alkyne Reactants
Alkyne + xs HX
Interconversion of Dihalides to Alkynes Transformation
Dihalide to alkyne
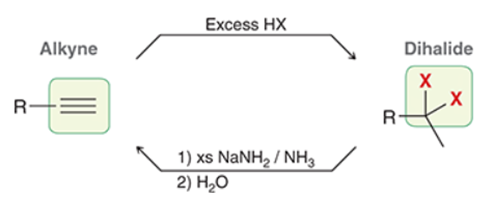
Interconversion of Dihalides to Alkynes Regiochemistry and Stereochemistry
R: N/A
S: N/A
Interconversion of Dihalides to Alkynes Reactants
Step 1: Dihalide + xs NaNH2 in NH3
Step 2: H2O
Radical Addition of HBr to Alkyne Transformation
Alyne to alkyl halide on alkene
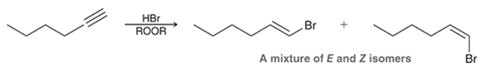
Radical Addition of HBr to Alkyne Regiochemistry and Stereochemistry
R: Anti-Markovnikov
S: Racemic
Radical Addition of HBr to Alkyne Reactants
Alkyne + HBr + ROOR (H2O2)
Acid-Catalyzed Hydration of Alkyne Transformation
Alkyne to ketone
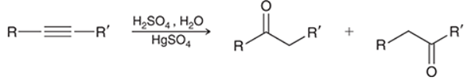
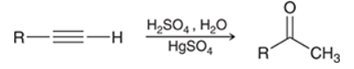
Acid-Catalyzed Hydration of Alkyne Regiochemistry and Stereochemistry
R: Markovnikov
S: N/A
Acid-Catalyzed Hydration of Alkyne Reactants
Alkyne + H2SO4 + H2O in HgSO4
Hydroboration-Oxidation of Alkyne Transformation
Terminal alkyne to aldehyde
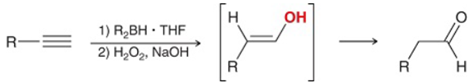
Hydroboration-Oxidation of Alkyne Regiochemistry and Stereochemistry
R: Anti-Markovnikov
S: N/A
Hydroboration-Oxidation of Alkyne Reactants
Step 1: Alkyne + 9-BBN or disamylborane
Step 2: H2O2 + NaOH
Tetrahalide Halogenation of Alkyne Transformation
Alkyne to tetrahalide on alkane
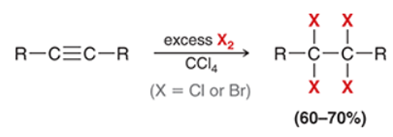
Tetrahalide Halogenation of Alkyne Regiochemistry and Stereochemistry
R: X adds to both sides of pi bond
S: N/A
Tetrahalide Halogenation of Alkyne Reactants
Alkyne + xs X2 in CCl4
Dihalide Halogenation of Alkyne Transformation
Alkyne to major trans dihalide on alkene and minor cis dihalide on alkene
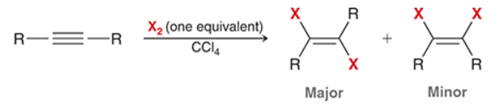
Dihalide Halogenation of Alkyne Regiochemistry and Stereochemistry
R: X adds to both sides of the pi bond
S: Anti major and syn minor
Dihalide Halogenation of Alkyne Reactants
Alkyne + 1 eq. X2 in CCl4
Ozonolysis of Alkyne Transformation
Internal alkyne to two carboxylic acids and terminal alkyne to a carboxylic acid and CO2
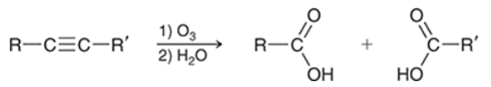
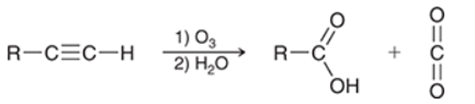
Ozonolysis of Alkyne Regiochemistry and Stereochemistry
R: Internal is not selective, terminal C-C becomes carboxylic acid and C-H becomes CO2
S: N/A
Ozonolysis of Alkyne Reactants
Step 1: Alkyne + O3
Step 2: H2O
Alkylation of Terminal Alkene Transformation
Terminal alkyne to a longer alkyne
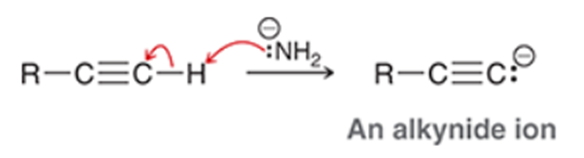
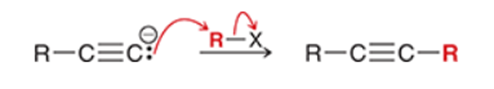
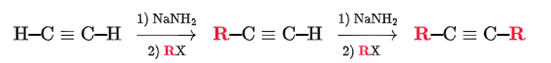
Alkylation of Terminal Alkene Regiochemistry and Stereochemistry
R: Must be side of C-H bond
S: N/A
Alkylation of Terminal Alkene Reactants
Step 1: NaNH2
Step 2: RX (R is methyl or ethyl)