1.5 Symbols, Formulae and Equations 🔣
1/40
Earn XP
Description and Tags
GCSE CCEA Specification GCSE Chemistry Double Award Science, Triple Award Science Unit 1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
Chemical symbol
represents element in periodic table with one or two letters, first being capital
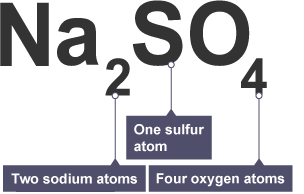
Interpreting formula
subscript refers to number of element before
Formula with brackets
multiply atoms inside with subscript outside
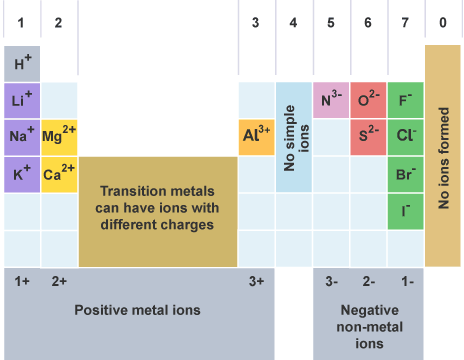
Ion charge/ valency
Group 1: +1
Group 2: +2
Group 3: +3
Group 4: ±4
Group 5: -3
Group 6: -2
Group 7: -1
Group 8: 0
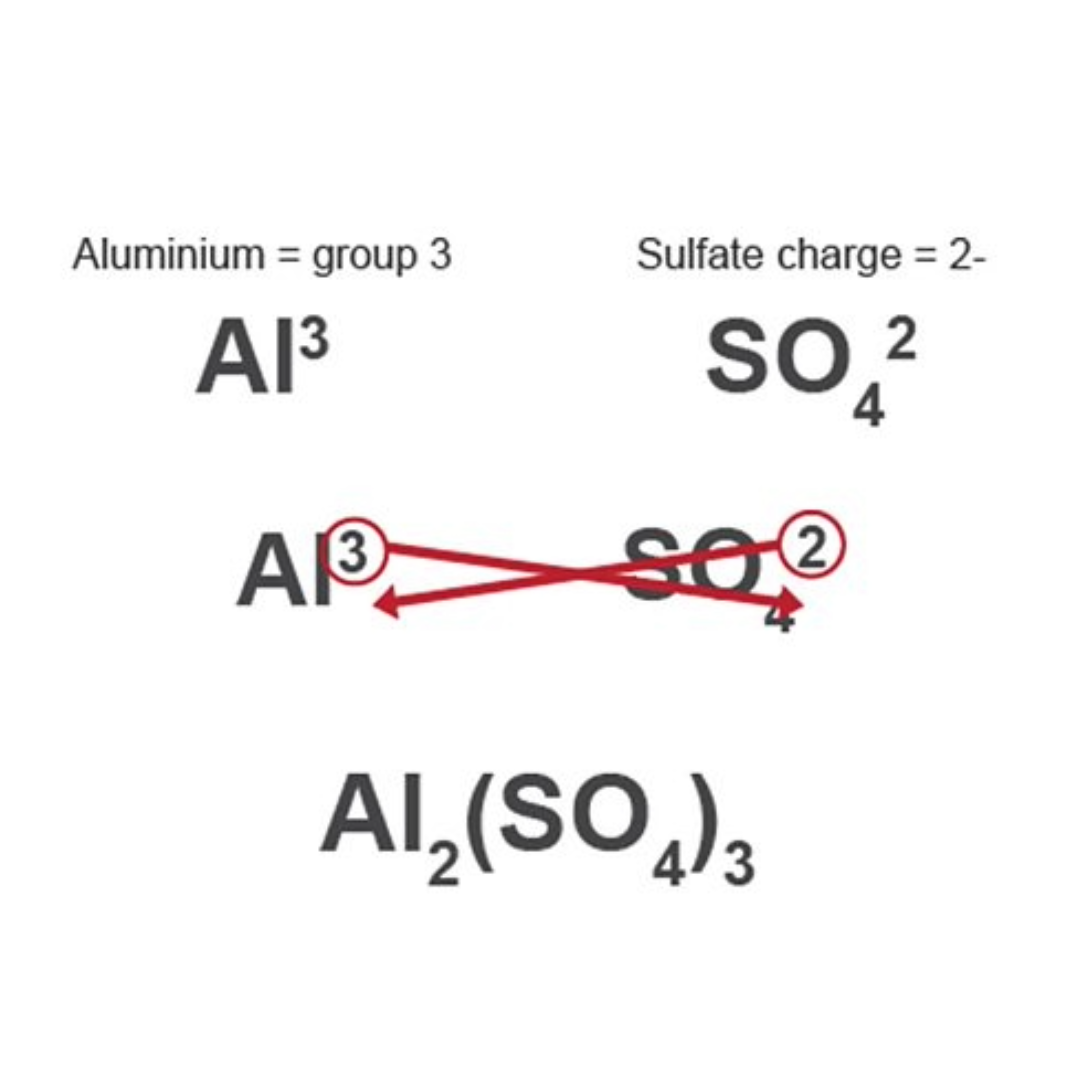
Working out formula (swap and drop)
write elements down with their charge/ valency
swap their numbers
write formula
Word equations
reactants → products
Metal + oxygen →
Metal oxide
Metal + cold water →
metal hydroxide + hydrogen
Metal + steam →
metal oxide + hydrogen
Metal + acid →
salt + hydrogen
Base + acid →
salt + water
Metal carbonate + acid →
salt + water + carbon dioxide
Metal carbonate →(heat)
metal oxide + carbon dioxide
Hydrocarbon fuel + oxygen →
carbon dioxide + water
Chemical reaction
rearranging atoms (reactants) to make new chemicals (products)
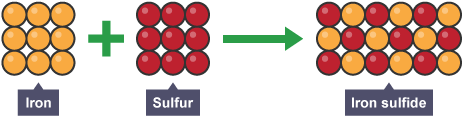
Conservation of mass
no atoms are lost or made but are rearranged, mass products = mass reactants
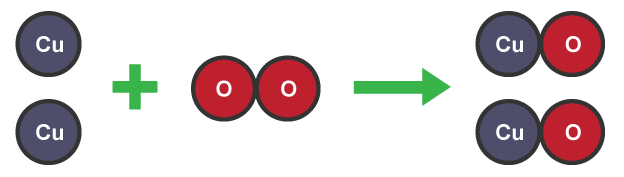
Balancing symbol equations
elements must have equal numbers of atoms on both sides
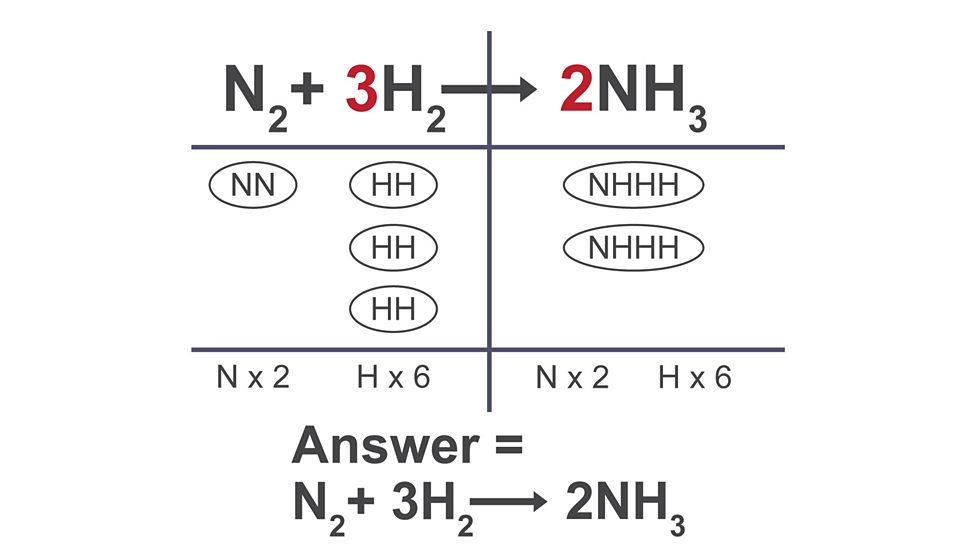
Writing balanced symbol equations
write out equation
count atoms on each side
add more until they are equal
write the amount as coefficient (big number)
(s)
solid
(l)
liquid
(g)
gas
(aq)
aqueous solution (dissolved in water)
Ionic equations
only shows ionic compounds which react, leaving out spectator ions
Neutralisation ionic equation (will always be the same)
H+(aq) + OH-(aq) → H2O(l)
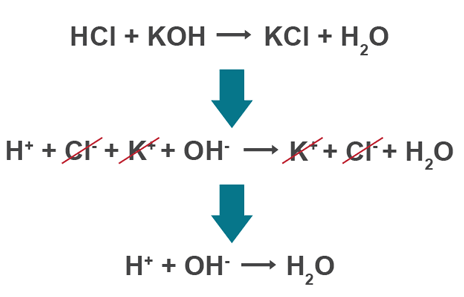
Displacement ionic equation
remove ions which do not react (one that switches)
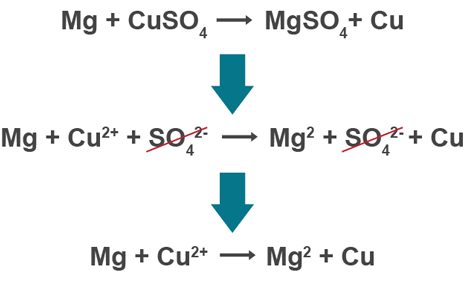
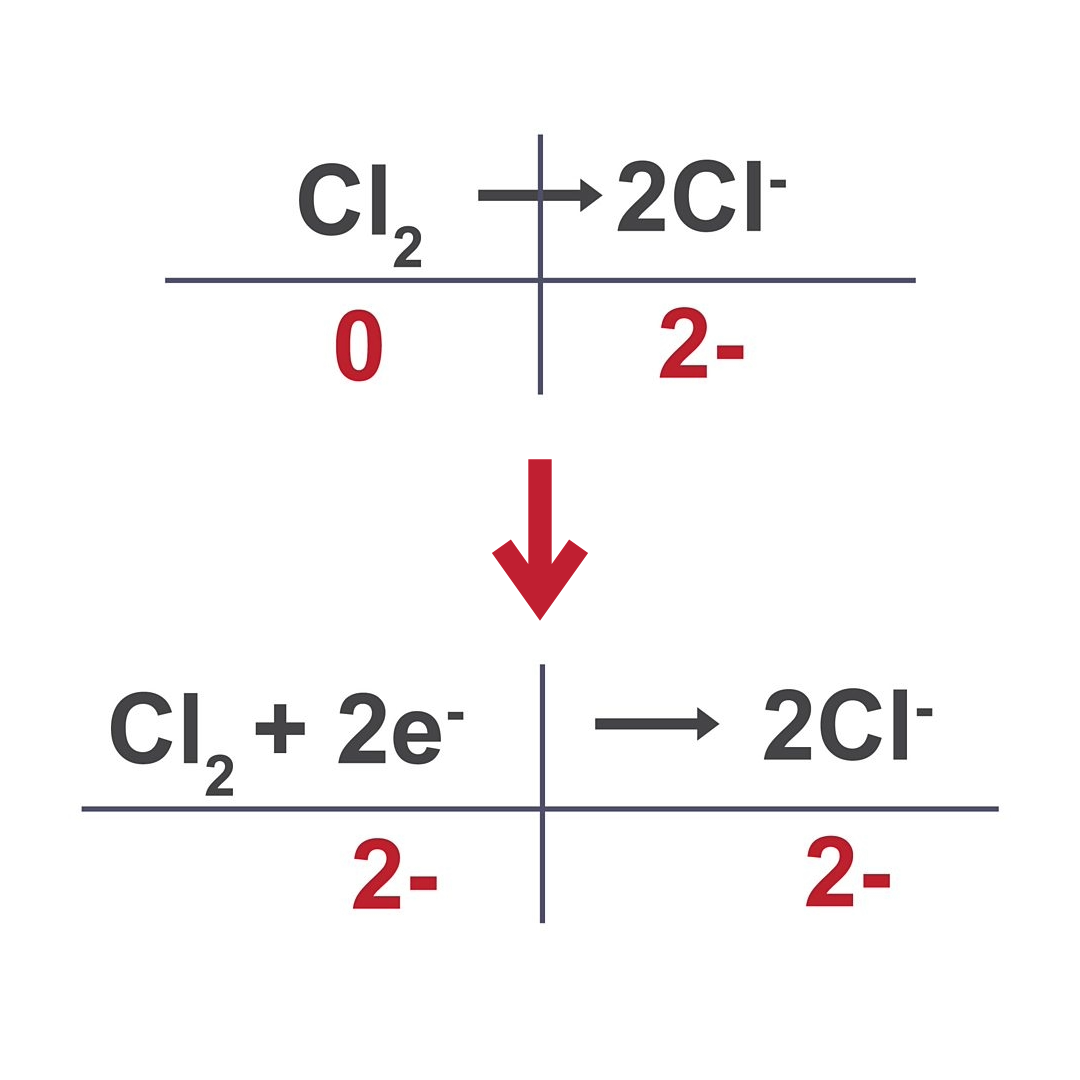
Half equations
shows change of atom/ molecule into ion
Writing half equations
write balanced reactant and product
write their charges
balance them by adding electrons
Common compounds
formula must be memorised as may not be given
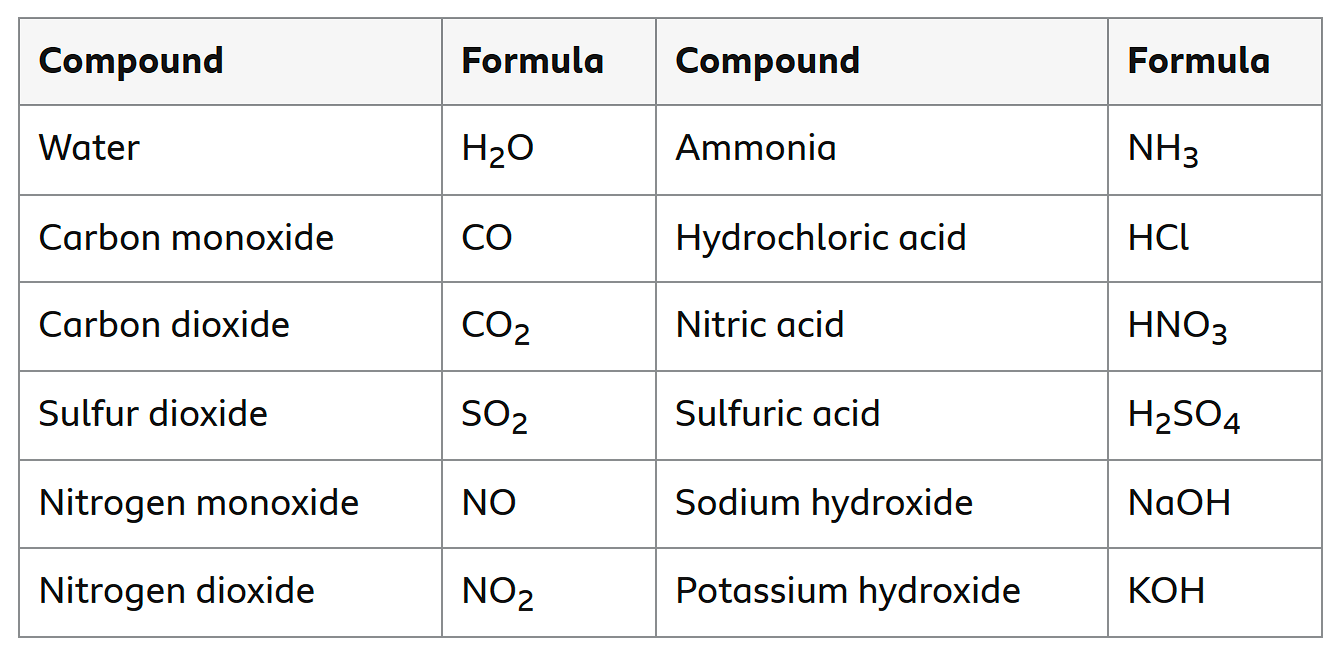
Water
H2O
Carbon monoxide
CO
Carbon dioxide
CO2
Sulfur dioxide
SO2
Nitrogen monoxide
NO
Nitrogen dioxide
NO2
Ammonia
NH3
Methane
CH4
Hydrochloric acid
HCl
Nitric acid
HNO3
Sulfuric acid
H2SO4
Sodium hydroxide
NaOH
Potassium hydroxide
KOH