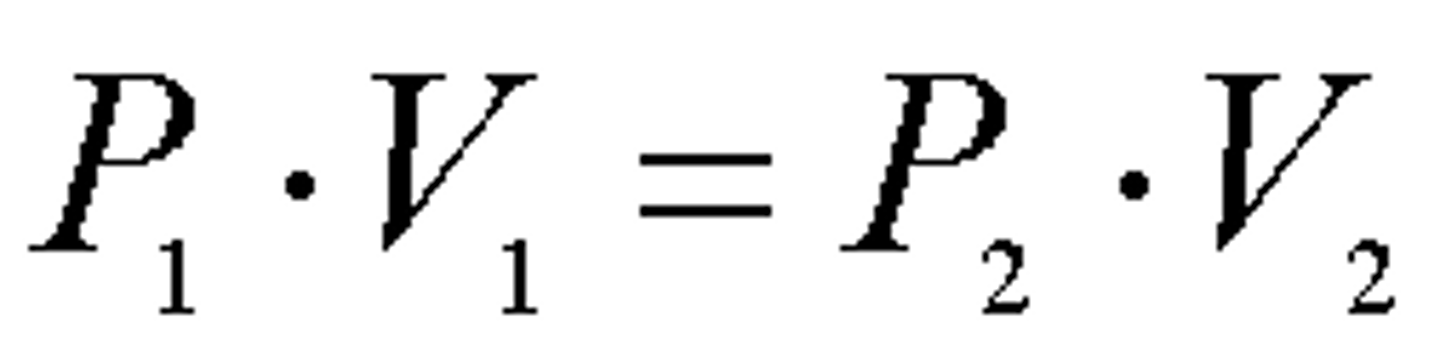pressure laws
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
inversely
volume is... related to pressure
on the numerator of the fraction (the top)
if the quantity in a ratio has to go up, where does the bigger number go
add 273
how do you make a temperature kelvin
directly
temperature is... related to pressure
directly proportional
number of moles is... to volume
equal numbers of moles
equal volumes of gas under the same set of conditions will contain...
0 degrees celsius & 1 atm
number of moles & volume are only proportional under the set set of conditions. these are:
Pv=nRT
whats the main formula for gas laws
ideal gas constant
what does the R stand for in Pv=nRT
increases
as volume increases, its' temperature... & vice versa
that volume & temperature are directly proportional
what does this formula show
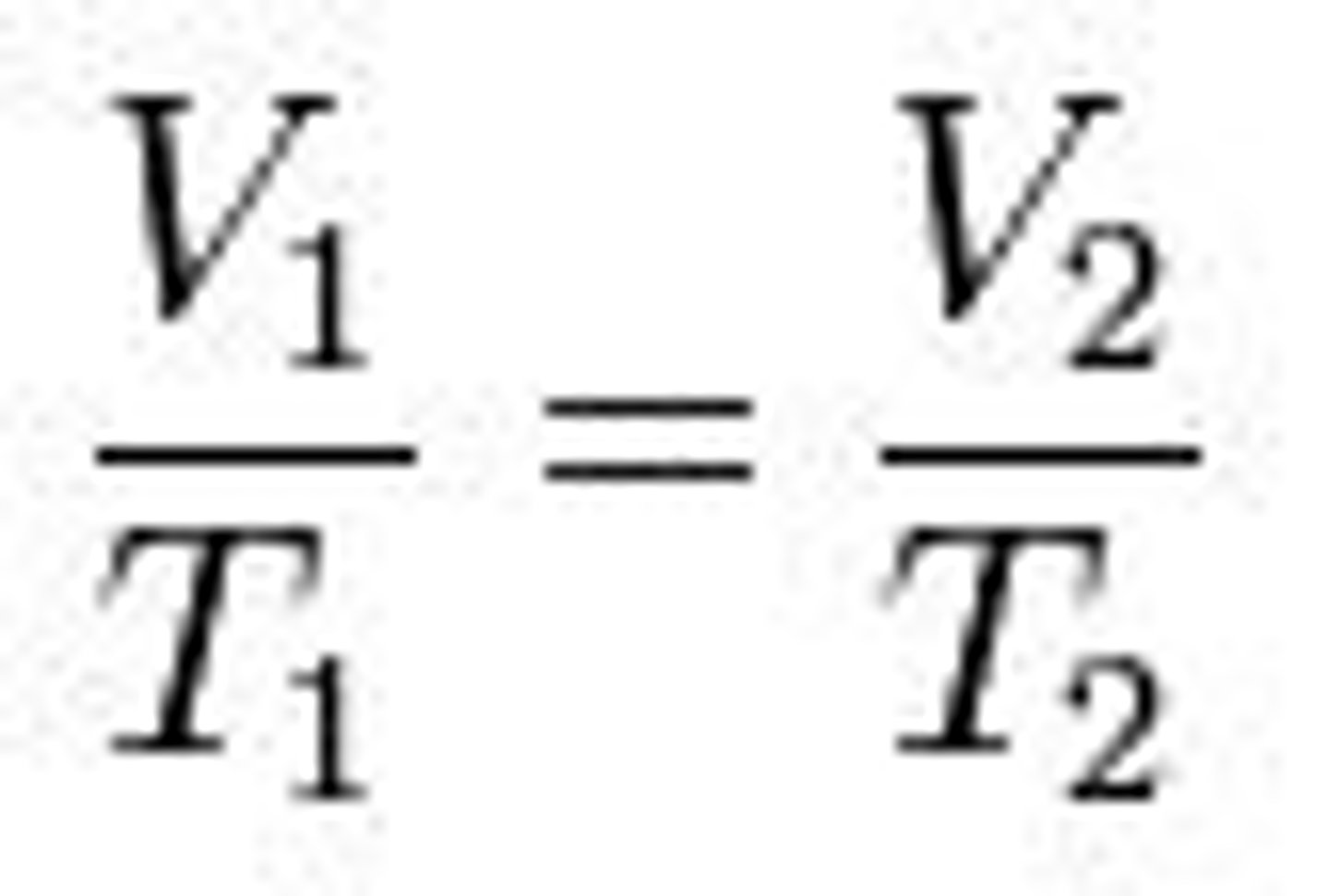
that volume is inversely related to pressure
what does this formula show