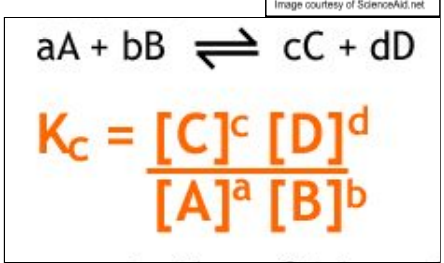AQA A LVL CHEM - equilibria extra
0.0(0)
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
what does increasing the concentration of reactants do to the position of equilibrium
shifts to favour the reaction that produces the products
(shifts to the right)
2
New cards
what does increasing the concentration of the products do to the position of equilibrium?
shifts to favour the reaction that (re-)produces/reforms the reactants
(shifts to the left)
3
New cards
do catalysts affect the equilibrium position?
what does it allow
doesnt affect the equilibrum position (affects both reactions equally)
allows equilibrium to be reached faster
4
New cards
how do u write Kc expressions (do one for the equation aA + bB → cC + dD)