Chem Scientists
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Democritus
All matter is made up of tiny particles that are indivisble
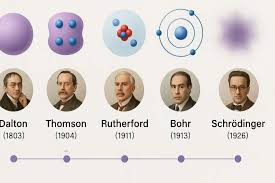
John Dalton
Common substances always broke down into the same elements in the same proportions
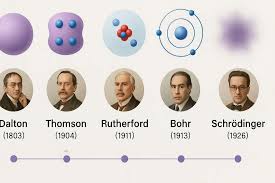
JJ Thompson
Atoms have negatively charged regions
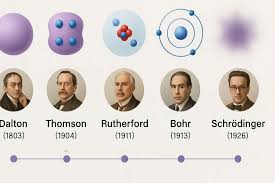
Ernest rutherford
Atoms consisted largely empty space with a positively charged nucleus in the center and electrons occupying the empty space around it
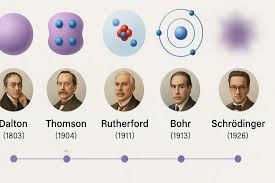
Niels Bohr
Built off of Rutherford, Electrons orbit the nucleus at fixed energies and distances able to jump from one level to another not the space between
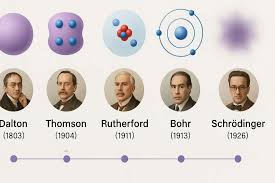
Erwin Schrodinger
Electrons exisit in organized orbitals called S,P,D,F
Louis de Brogile
electrion movement can be described as waves with wave like motion