substitution and elimination
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What are the six common nucleophiles?
I-
Cl-
Br-
HO-
HS-
N triple bond C-
Don’t need to be dully negative and can be partially rich
What are five common leaving groups?
I-
Cl-
Br-
Sulfuric acid
H2O
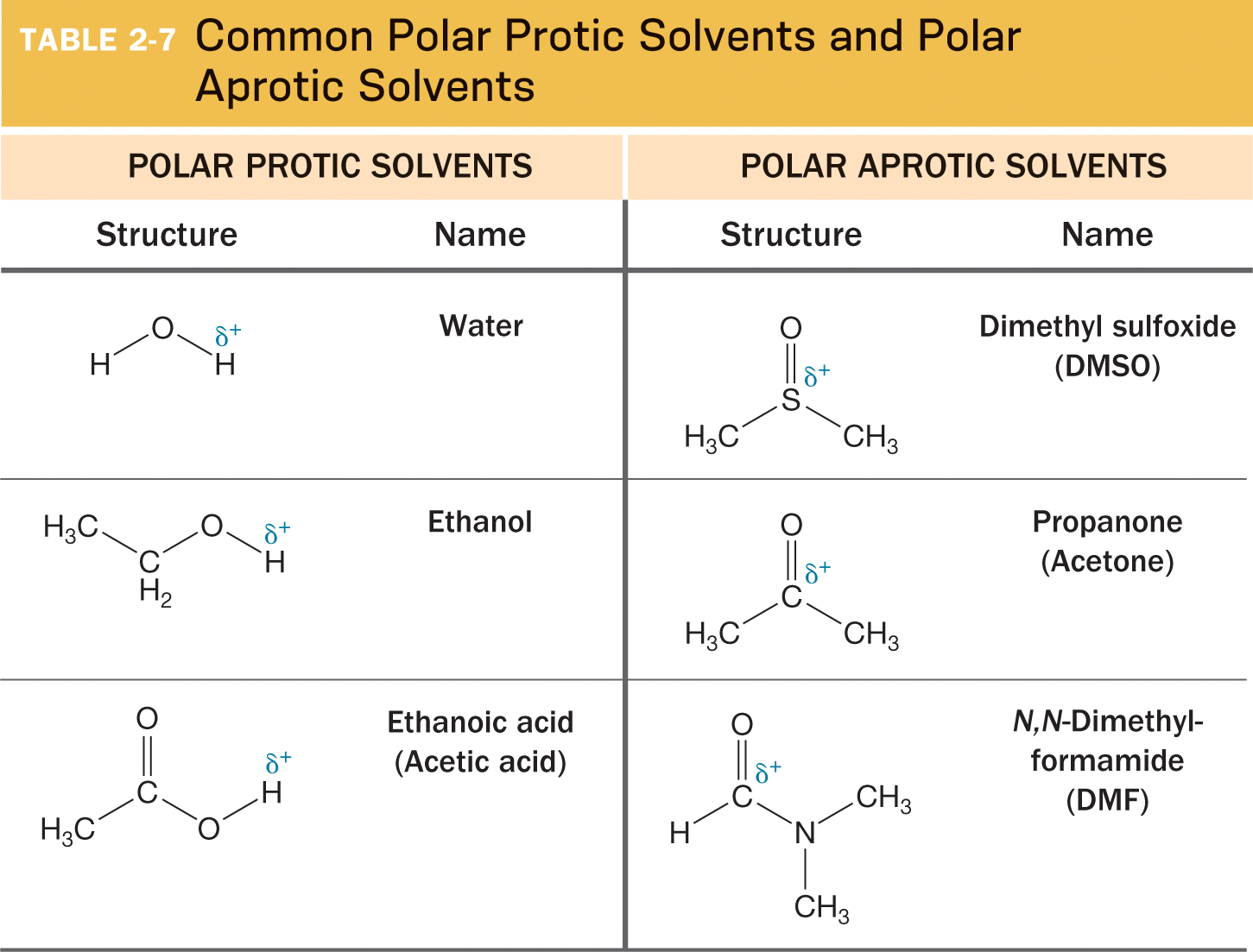
Nuclephiles strength ____ from right to left on a periodic table in oth polar protic solvents and polar aprotic solvents
increases
In a polar protic solvent, nucleophiles strength _____ a group. Why?
Increase down because larger atoms are more polarizable. The larger the atomic radius, the stronger the nucleophile.
What are five common polar protic solvents?
Water, methanol, ethanol, acetic acid, and ammonia
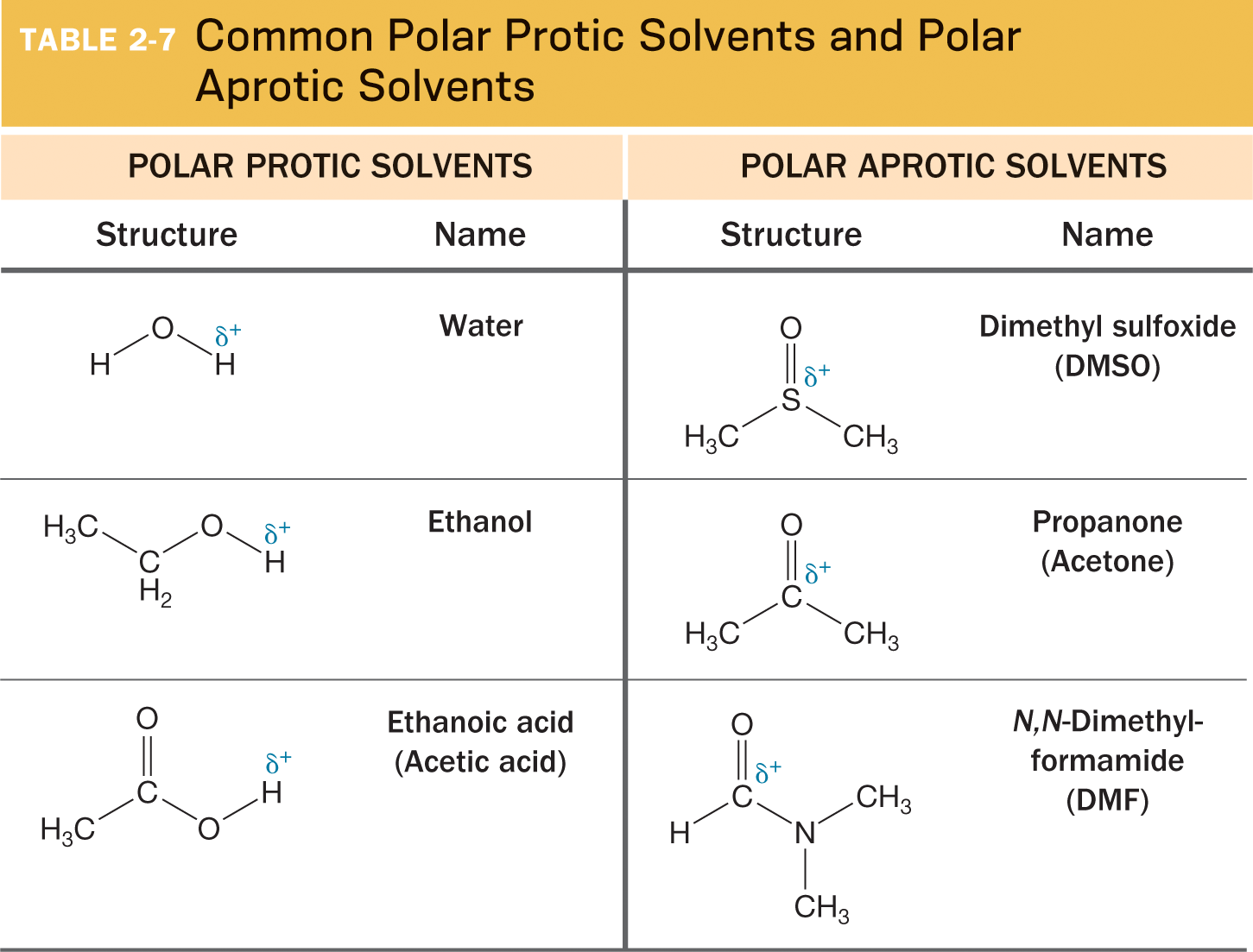
In a polar aprotic solvent, nucleophilicity ____ a group. Why?
decreases up the group due to lack of hydrogen bonding, which allows smaller nucleophiles to be more effective.
What are the four common polar aprotic solvents?
Acetone, DMSO, DMF, and THF
In an elimination reaction, a ___ is formed via the loss of a proton and a leaving group.
Double bond
In substitution reaction, a _____ is replaced with a nucleophile
leaving group
What occurs in a SN2 reaction?
A nucleophile attacks a substrate, displacing a leaving group in a single concerted step.
Due to SN2 reactions being concerted, they have a ?
A pentavalent transition state and a bimolecular rate law.
What is a pentavalent transition state?
It consists of the electrophile weakly coordinated to both the nucleophile and the leaving group
In an SN2, does the nucleophile attack the electrophile from the back or the front?
Back
SN2 reactions are stereospecific. If a nucleophile attacks a chiral center, what happens?
An inversion of stereochemistry occurs. For example if it was R then it will be S
Is SN1 a one or more step process?
It is a more steps
What is the first step for SN1 reaction?
It is a carbocation intermediate formation via the loss of a leaving group. This is considered as the rate limiting step of the reaction
What occurs in the second step for SN1 reaction?
Is a carbocation rearrangement, if possible
What is the final step of SN1?
It is a nucleophile attack of the carbocation to form the final product.
SN1 can only occur in what type of substrate?
Only secondary and tertiary substrates are required because they require stable carbocations for the reaction to proceed.
SN1 reaction is a ___ rate law and does not include the?
Unimolecular rate kaw and does not include the concentration of the nucleophile.
What does the Zaitsev product refer to?
To the more substituted alkene
What does the Hofmann product refer to?
Less substituted alkene