paper 1 chem
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
explain chelate effect
1) multidentate ligands replace monodentate ligands
2) because there is an increase in entropy
graph for how S2O82- ions change over time
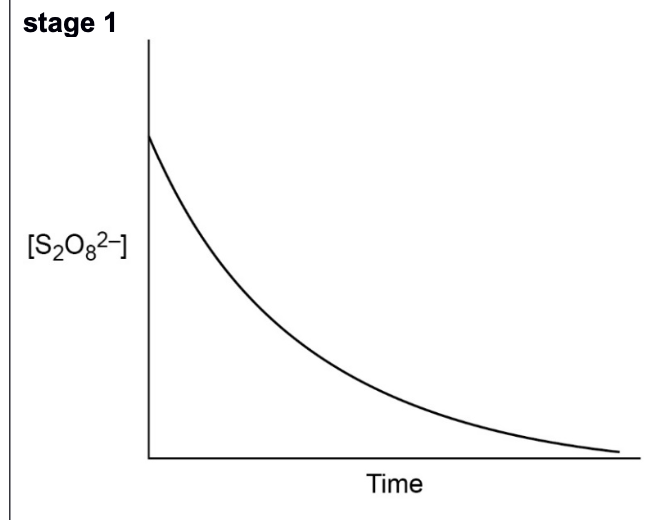
explain how fe2+ catalyse the reaction
2a (reaction slow) because S2O82– and I– repel/high
Ea
Or
(reaction slow) because two negative ions repel/high
Ea
2b Fe2+ attracts the S2O82– so lower Ea
Or
Fe2+ and S2O82– oppositely charged so lower Ea
2c Iron/Fe has a variable oxidation state
colour of iron carbonate
GREEN
role of sulfuric acid with nacl
PROTON ACCEPTOR
why does the second electron affinity of oxygen have a positive value
(O-) negative ion repels electron
explain why the enthalpy of lattice dissociation for sodium oxide is greater than the enthalpy of lattice dissociation for sodium chloride
oxide ions have a higher negative charge stronger attraction between the oppositely charged ions
why is there not a data book value for the enthalpy of solution of sodium oxide
sodium oxide reacts with water to produce NaOH
why do you use a stoppered flask after filtration
to prevent reaction with CO2
to prevent evaporation of water from the solution
state the meaning of a transition metal complex
(Central) metal atom/ion surrounded by ligand
suggest why … is neutral
ligand neutral + complex is neutral
example of e/z isomerism
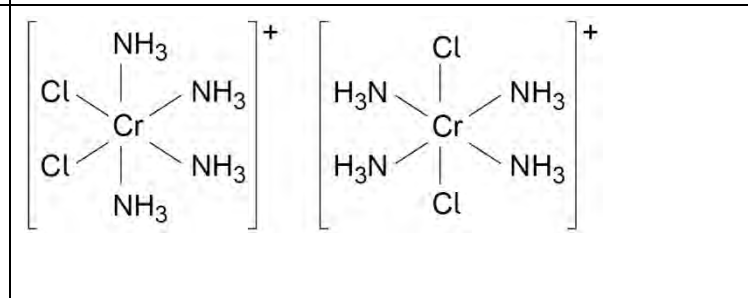
effect on emf if water added to rhs electrode
emf increases
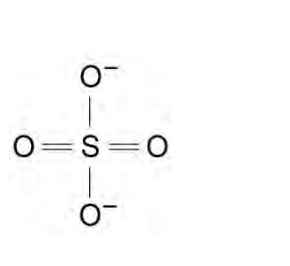
displayed formula for SO42-
describe how solution is made up to 250 cm³
M1 Transfers the solution to a volumetric/graduated flask
M2 Add washings using distilled water and make up to the graduation mark/250 cm3
M3 Invert many times / shake to mix
why is it good practice to rinse pippete with some alkali
So that the titration is done with known concentration of alkali
Allow so that water does not dilute the solution
aluminium sulfate and water
Al2(SO4)3 + 12 H2O → 2 [Al(H2O)6] 3+ + 3 SO4 2–
aluminum hexa aqua copper and excess ammonia
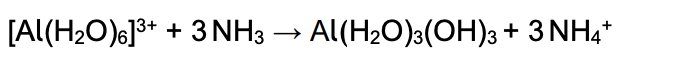
methanol and water half equation
CH3OH + H2O —> O2 + 6H+ + 6 e-
why does a fuel cell not need to be electrically charged
Reactants supplied continuously
advantage of using methanol over hydrogen
Methanol (is liquid so) can be stored easily or transported easily
how is relative abundance determined TOF
CURRENT is proportional to abundance
explain why titration cannot be done using an indicator
weak acid weak base titration
ph change is too gradual (no vertical section of graph)
perfect ionic model smaller than calculated value
some covalent character
the bonds holding the lattice together are stronger
describe a test between sodium oxide and phosphorus oxide
1) add water
2) add blue litmus paper
3) blue with sodium oxide and red with phosphorus oxide
copper hexa aqua 2+ and excess ammonia equation
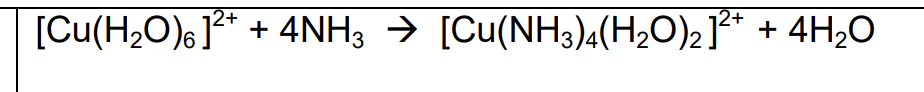
why can calorimetry not be used to determien conc of solutions containing (cucl2)-
1) has a full 3d subshell
2) cannot absorb frequencies of visible light/ it is colourless
why is enthalpy change for chelate effect close to 0
SAME number and TYPE of bond being broken and made