topic 5- energy changes
1/43
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
what is an exothermic reaction?
A reaction that transfers energy from the reaction molecules to the surroundings
What happens to the temperature of the surroundings around an exothermic reaction?
Temperature of the surroundings increases
an example of types of exothermic reactions?
combustion (burning fuels)
neutralisation reactions
many oxidation reactions
examples of exothermics every day uses?
hand warmers
self heading cans
what would an energy profile diagram look like for an exothermic reaction?
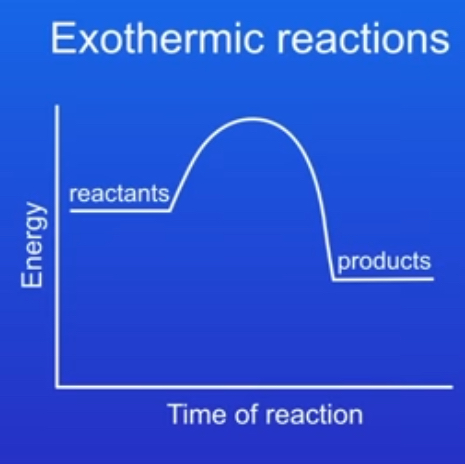
why did the products have less energy than the reactants in an exothermic reaction?
Because energy has been transferred from the reaction to the surroundings
what are endothermic reactions?
endothermic reactions are ones that take in energy from the surroundings
what are some types of endothermic reactions?
reaction between citric acid and sodium hydrogen carbonate
thermal decomposition
What happens to the temperature of the surroundings around an endothermic reaction?
The temperature of surroundings decrease
what would the energy profile of an endothermic reaction look like?
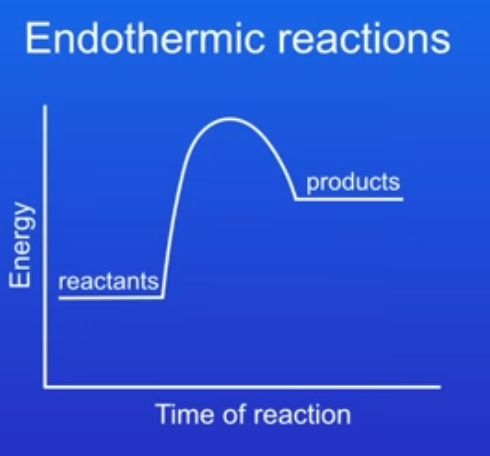
examples of uses of endothermic reactions?
sports injury packs No
what is the definition of activation energy?
The minimum amount of energy that particles must have to react
what happens to the bonds in an endothermic reaction?
bonds break
why do the bonds break in an endothermic reaction?
energy must be supplied to break existing bonds
what happened to the bonds in an exothermic reaction?
bonds form
why are bonds formed in an exothermic reaction?
Because energy is released
How would you carry a practical to determine temperature change?
Use a measuring cylinder to measure 30 cm³ of dilute hydrochloric acid
Transfer the acid into a polystyrene cup
stand the Polystyrene cup inside a beaker (stops the cup from falling over)
Use a thermometer to measure the temperature of the acid
use a measuring cylinder to measure 5 cm³ of sodium hydroxide solution
transfer this into the polystyrene cup
fit plastic lid onto the cup and place the thermometer inside
use a thermometer to gently stir the solution
because the reaction is exothermic (neutralisation reaction), it will release energy so the temperature of the solution will increase
Record the temperature reached
Now rinse out and dry the polystyrene cup
Repeat the whole experiment with using 10 cm³ of sodium hydroxide solution
do this multiple times with increasing the solution by 5 cm³ each time
once you have done this, repeat the whole experiment again, so you have two sets of results
you can then find out on me for each volume of solution
you can then plot a graph of your results
what would the graph of your results look like?
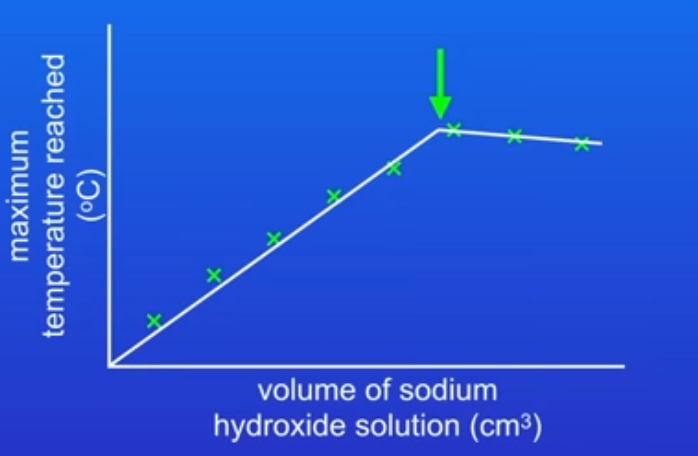
why after a certain point does the maximum temperature reached start to decrease?
Because as you add a greater volume of solution so the energy release is now spread into a greater volume.
why is a polystyrene cup used?
It’s a good thermal insulator so it reduces heat loss
why do you put a lid on the top?
Reduces heat loss to the air
what is an electrochemical cell?
A basic system made up of two different electrodes in contact with an electrolyte
why are the electrodes usually metals?
Because they must be able to conduct electricity
what is the electrolyte?
A liquid that can conduct electricity (contains ions which react with the electrodes)
What happens when we take two different metals and placed them into an electrolyte?
We can produce electricity
what does an electric cell look like?
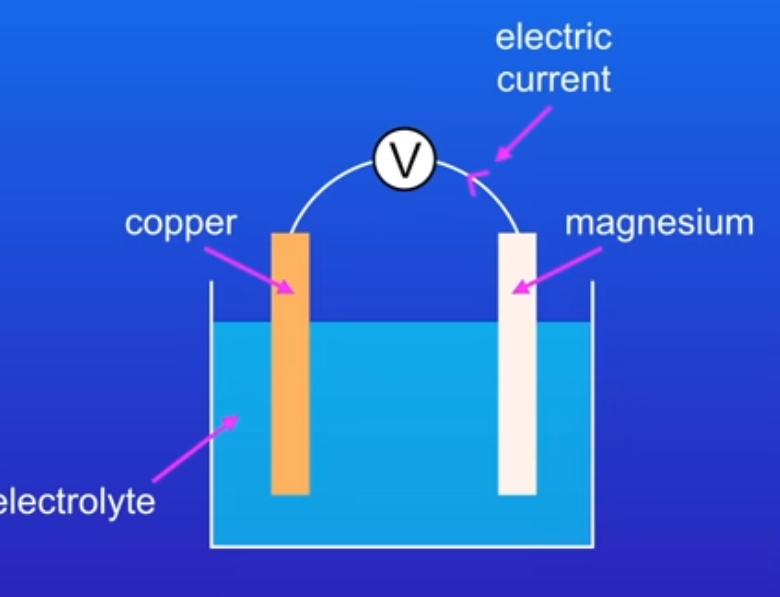
why can a cell only produce electricity for a certain amount of time?
Eventually, the chemicals in the cell run out and the reaction stops
what must the metals be to produce electricity?
Must use metals with different reactivities
what happened when the electrodes get connected by a wire?
The charge is able to flow and electricity is produced
what is the size of the potential difference depend on?
The difference in reactivity between the two metal
what is a battery?
Contains two or more cells connected in series to produce a greater voltage
Image of a battery?
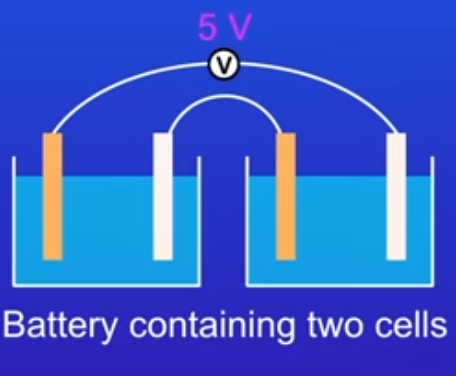
why do batteries run out?
Because the reactants in the batteries run out so no more electricity is produced (irreversible)
examples of batteries that can run out
alkali batteries
why can rechargeable batteries be recharged?
Because the chemical reactions can be reversed
what do fuel cells generate?
electricity
what happens in a fuel cells?
we react a fuel with either pure oxygen or air, inside the fuel cell a chemical reaction takes place which produces an electrical current
what is the only waste product from a fuel cell?
water
diagram of a fuel cell?
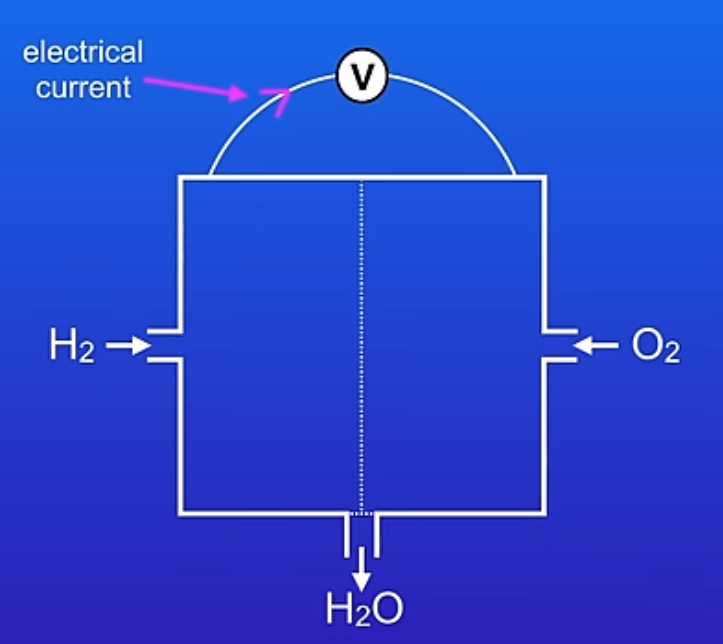
what is the advantage of fuel cells in terms of their life span compared to rechargeable batteries?
hydrogen fuel cells will produce electricity for as long as you provide hydrogen
Rechargeable batteries run out and need to be recharged.
what is the advantage of fuel cells producing water as a waste product?
• Hydrogen fuel cells can be a source of drinkable water eg on space-craft. |
disadvantages of fuel cells using the gas hydrogen?
• Hydrogen fuel cells run on hydrogen which is an explosive gas and is very difficult to store safely.
what is the difference in potential difference produced between fuel cells and rechargeable batteries?
Hydrogen fuel cells produce a relatively low potential difference or voltage so several are needed together.
• Rechargeable batteries can produce a greater potential difference than a hydrogen fuel cell.