Biopharmaceutical Chemistry - 5
1/76
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
77 Terms
Structure of amino acids
o Amine group, Carboxyl group
o Both are attached to the same carbon (α-carbon)
o R: 20 different groups for proteinogenic amino acid
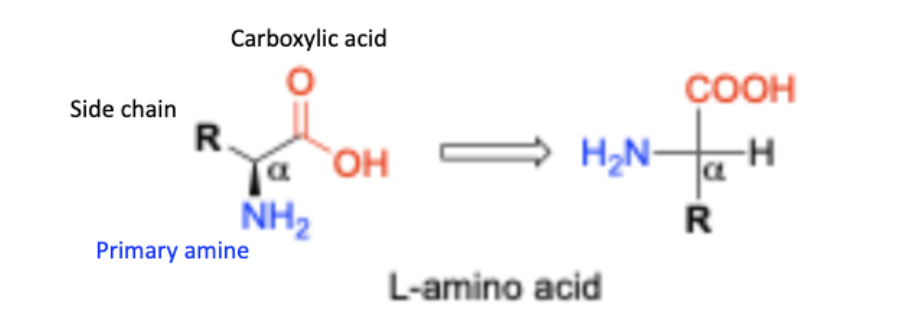
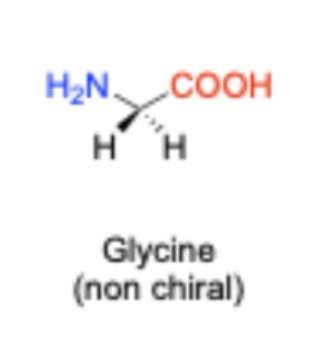
Are all amino acids chiral?
All except for one (glycine is the exception)
Glycine
Amino Acids and polarity
Can be polar, non-polar, and neutral
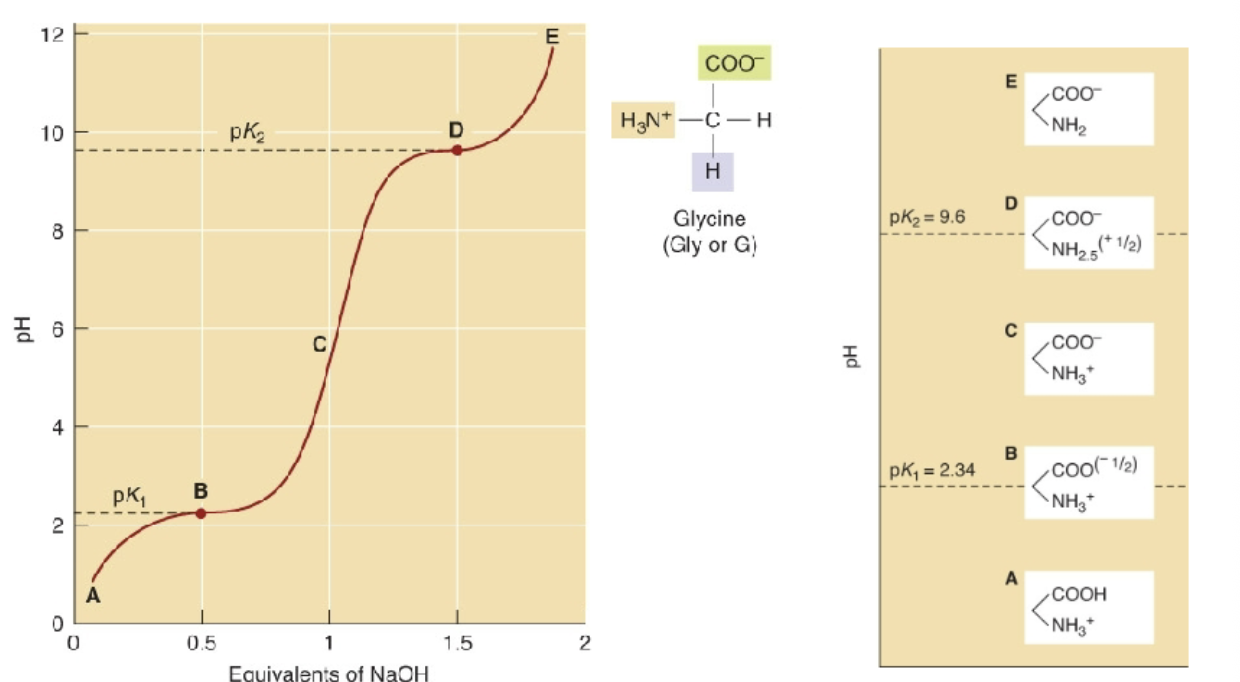
Acid Base Properties
There are multiple dissociable groups in a single molecule
Two equivalents of NaOH are needed to remove both protons
Number of Buffering regions in Glycine
There are two buffering regions
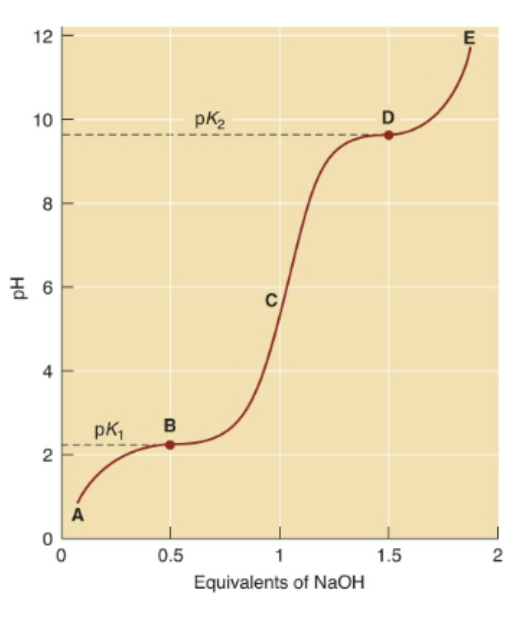
Acid-Base Properties in comparison to charge
pK2 of 9.6 is where fully deprotonated.
pK1 of 2.34 is where still completely protonated.
Zwitterions
Two charged groups, but the molecule has an overall charge of zero
o How most amino acids exist at physiological pH
o Favored due to electrostatic interactions between the amine and CO2 groups
Why is there a difference in pKa?
Due to the through-space effect
• Carboxyl and amine attached to same carbon
• Due to attraction of opposite charges
What is the isoelectric point (pl)?
The pH at which the zwitterion has exactly a zero charge
Equation for pl:
(pK1 + pK2)/2
Example of Multiple Dissociation:
Lysine
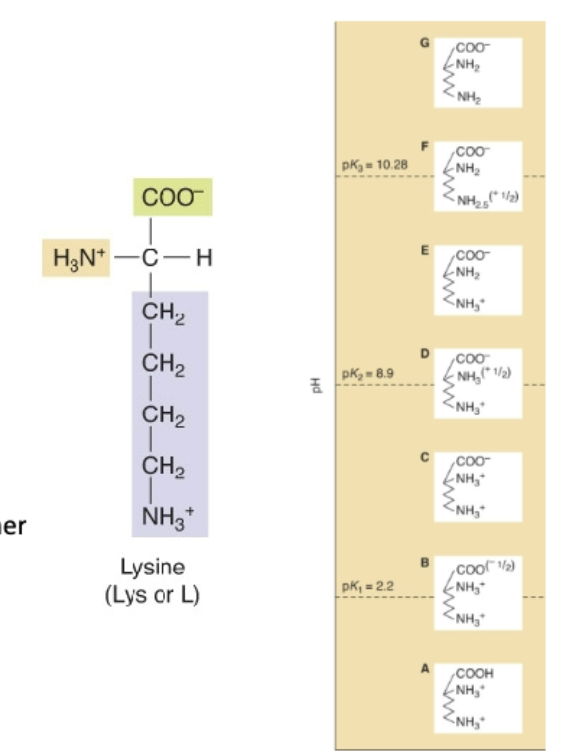
What happens when there is multiple dissociation groups (more than two)?
Challenging to identify buffering regions
We can have more than 3 charges
How do we determine the pl with multiple dissociable groups?
Determine zero charge
o Average 2 pKa values surrounding it
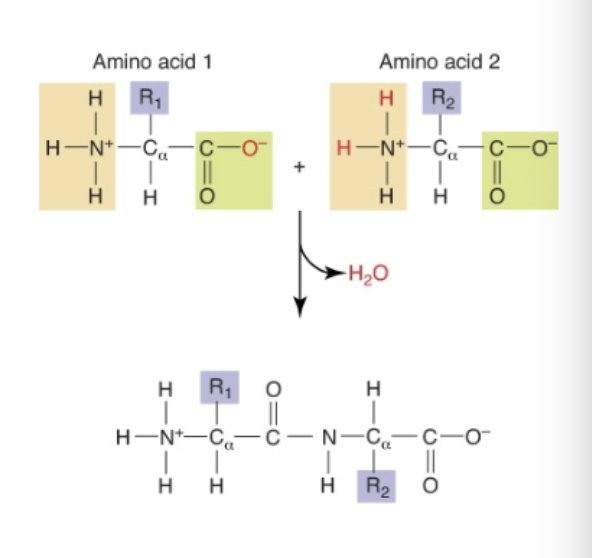
What is a Peptide Bond?
Covalent link between α-amine of one amino acid with α-carboxyl group of another
How are peptide bonds formed?
By dehydration
Why are peptide bonds stable?
Resonance increases the stability of the peptide (amide) bond and makes it rigid.
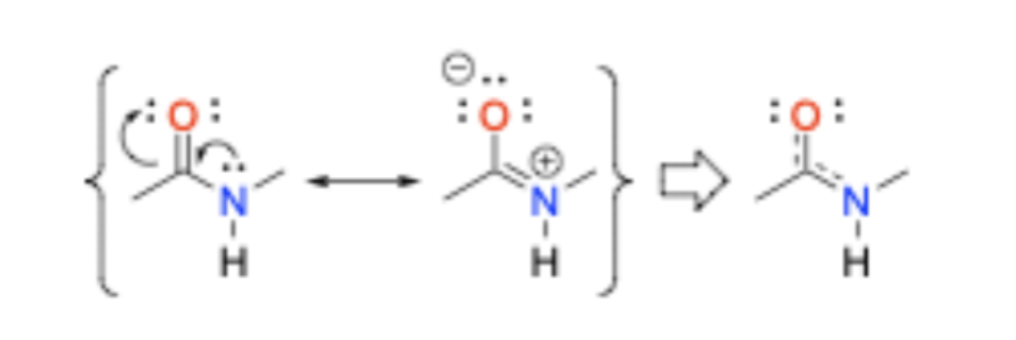
Are amides or esters more stable?
Amides are more stable than esters towards hydrolytic cleavage
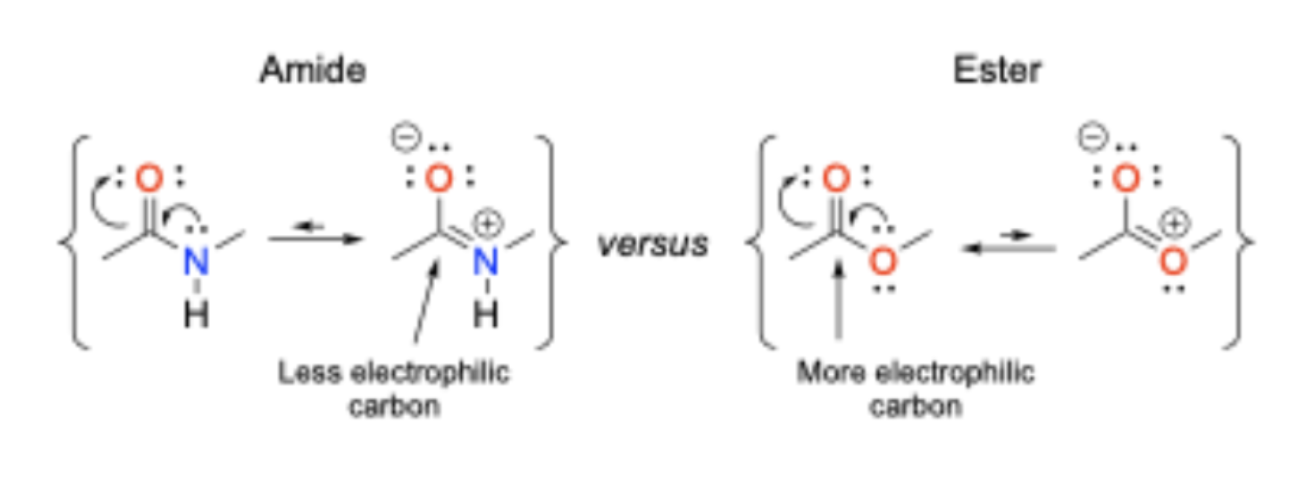
Rotation on peptide bonds
Free rotation about peptide bonds is restricted
• Protein backbones are more rigid than flexible
• More stable conformations
What are amino bonds considered as?
neutral functional groups
What are peptides?
Amino acid oligomers of <25 amino acids.
Peptides act:
Behave like small molecules
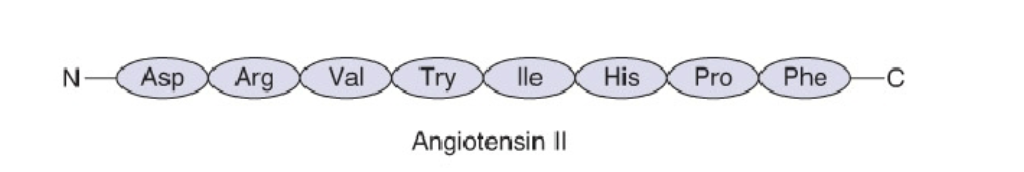
o Angiotensin II: Linear (cause vasoconstriction and increased blood pressure)
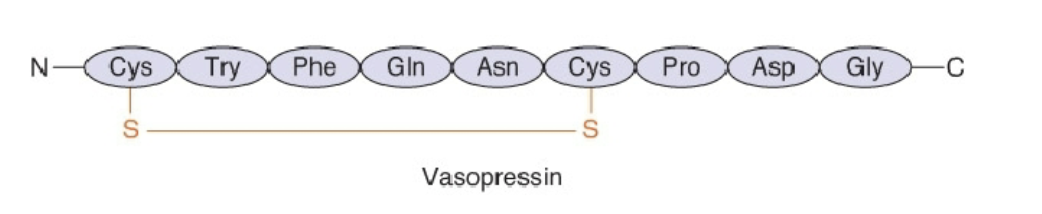
o Vasopressin: Cyclic peptide, constrained, compact (constricts blood vessels, retain water)
Angiotensin II
Linear (cause vasoconstriction and increased blood pressure)
Vasopressin
Cyclic peptide, constrained, compact (constricts blood vessels, retain water)
What are proteins?
Amino acid oligomers of <25 amino acids.
Proteins act:
Distinctive polymer behavior
o Involved in critical biological functions
o Most proteins are labile to temperature, pH, and ionic force.
How are proteins metabolized?
by other proteins called proteases (enzymes that hydrolyze peptide bonds)
Levels of Protein Structure
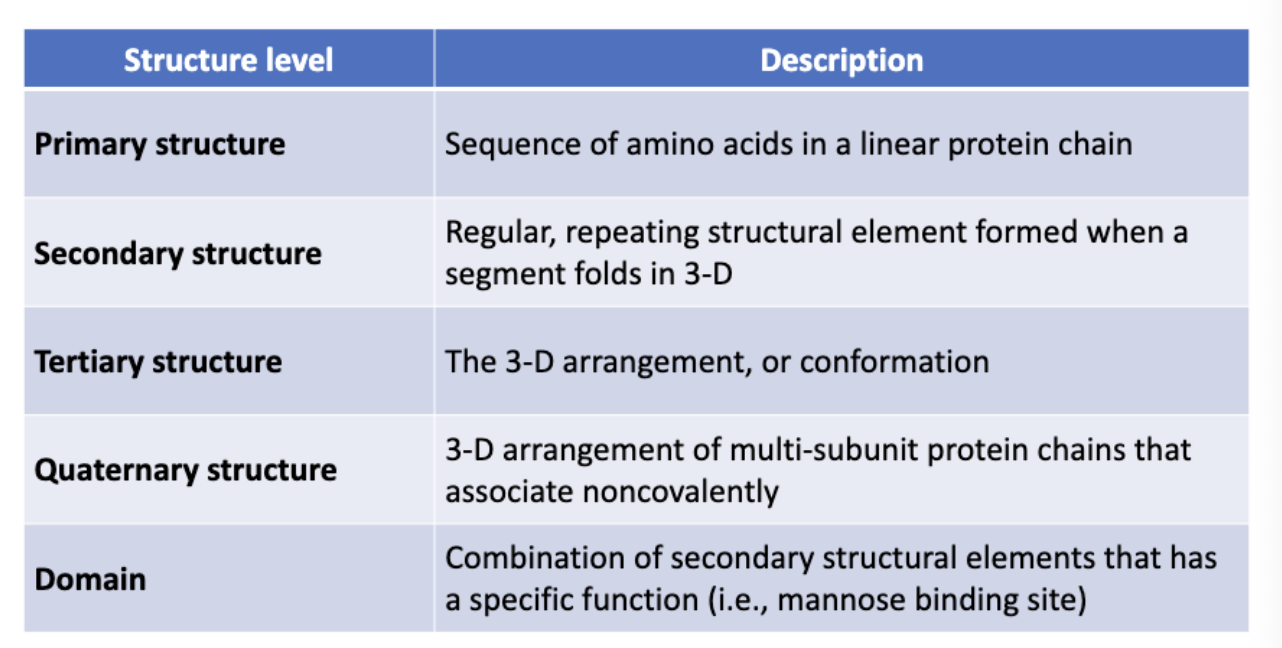
Primary structure:
Amino acid sequence that is encoded in the genome.
Is it possible to predict the protein’s three-dimensional shape from the amino acid sequence?
Yes, through Al AlphaFold
How can protein sequences be determined experiementally?
using proteases and chemical degradation in combination with analytical techniques like mass spectrometry.
Secondary Structures:
Loop or random coil: indeterminate in structure and link secondary structures together.
α-helix and β-sheet
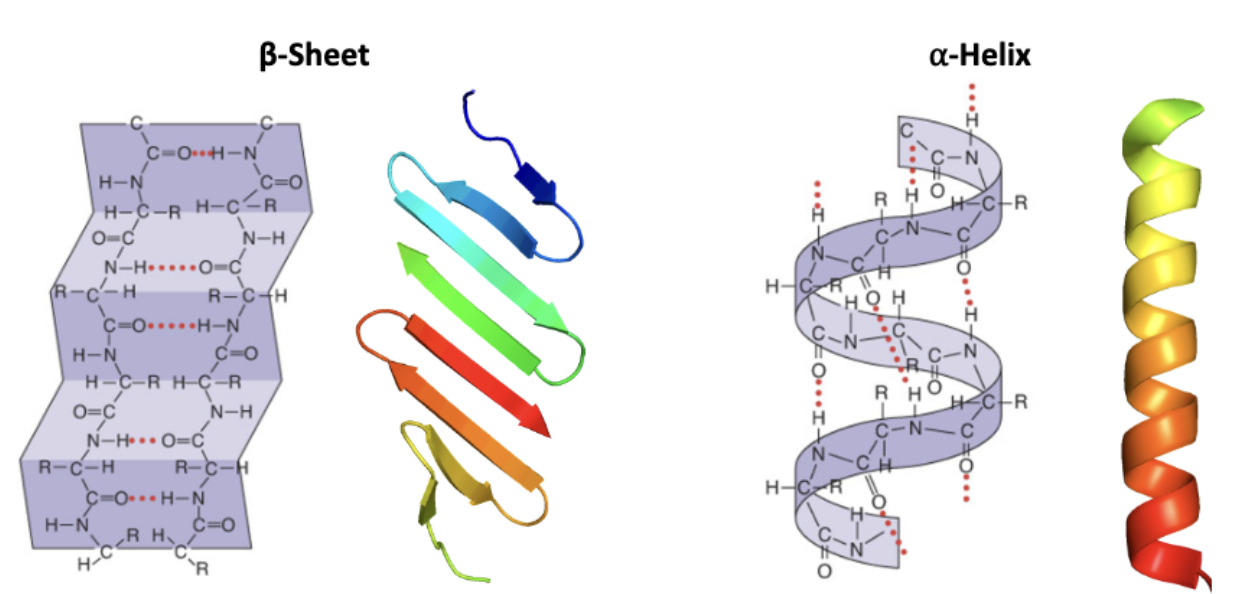
α-helix
H-bonds between N and O atoms
• Inner core = hydrophobic (No H-bonds available for water (involving peptide backbone))
• Water solubility determined by R groups
• Can be embedded in membranes when R groups are hydrophobic
Why is the inner core of α-helix hydrophobic?
H-bonds available for water (involving peptide backbone)
How does the R groups influence the α-helix formation?
Induce helix formation
o Hydrophobic amino acids
o Side chains with isolated charges
Prevent helix formation (helix breakers)
o Neighboring charges (electrical repulsion)
β-sheet
Stabilized by H-bonds between the backbone peptide bonds
o Need a turn to allow folding back on itself
These amino acids do not H-bond
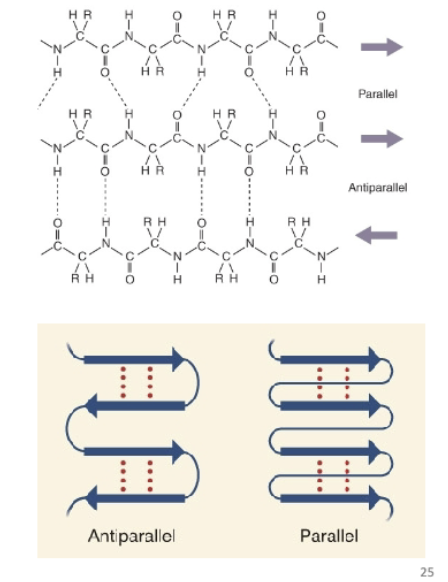
What are the two different alignments of the β-sheet?
Parallel and Antiparallel
Tertiary Structure
Three-dimensional structure of entire protein
Includes all aspects of lower-level structures
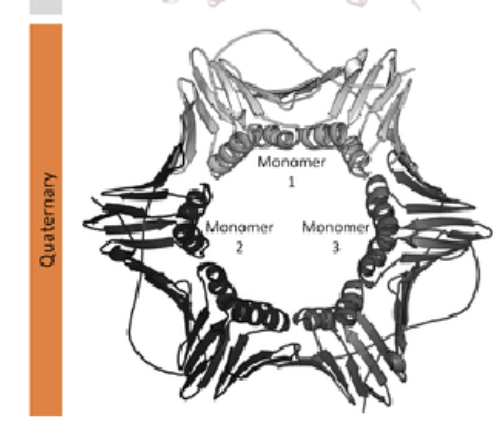
Quaternary Structure:
Proteins that consist of more than one chain
Made up of monomer subunits (individual protein chains)
o Bound noncovalently
• Charge or hydrophobic interactions
o Can be identical (i.e., Hsp90)
Domain
Structures associated with function; Exist between secondary and tertiary levels of structure
Most domains perform a specific job, such as binding a particular molecule (e.g., DNA, lipids, or ions) or catalyzing a chemical reaction.
Examples of Domains:
Triose phosphate isomerase (TIM domain); SH2 and PTB domain; Nicotinamide adenine dinucleotide (NAD) binding domain
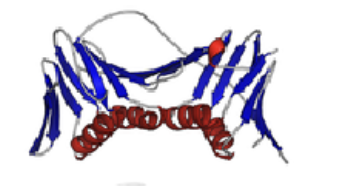
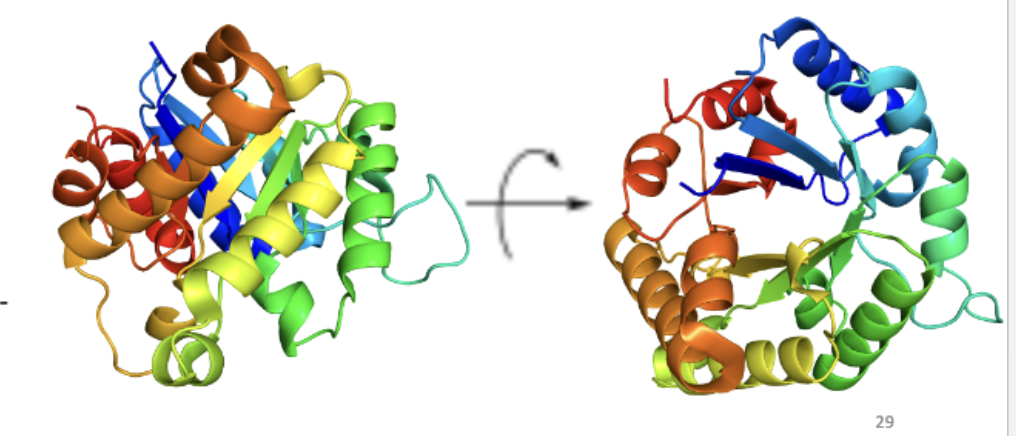
Triose phosphate isomerase (TIM domain)
Donut-shaped sequence
Inner portion: beta-sheet; Outer portion: alpha-helix
Catalyzes isomerization of dihydroxyacetone phosphate (DHAP) and D-glyceraldehyde-3- phosphate (G3P)
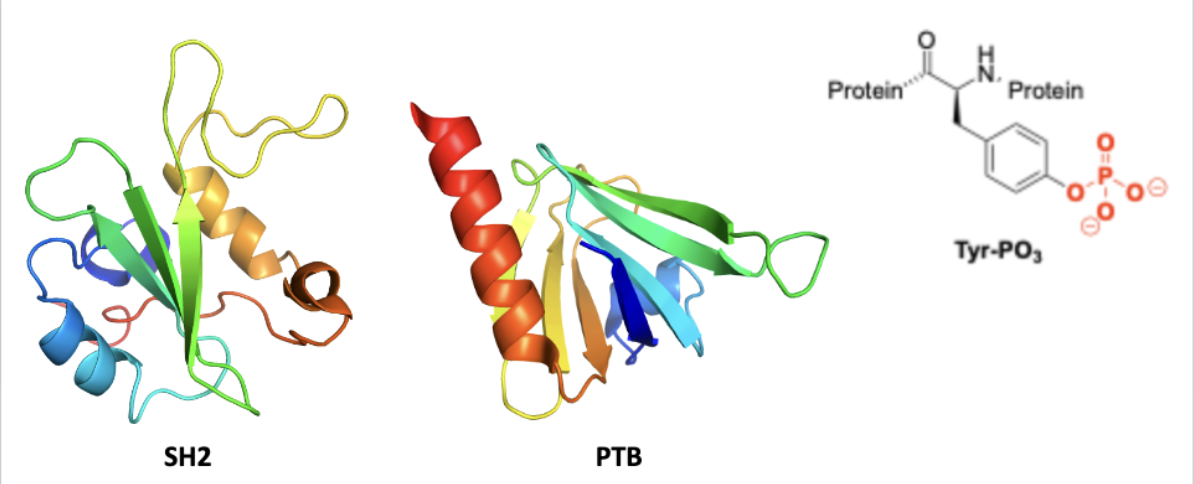
SH2 and PTB domains
bind phosphotyrosine (signal transduction ligand)
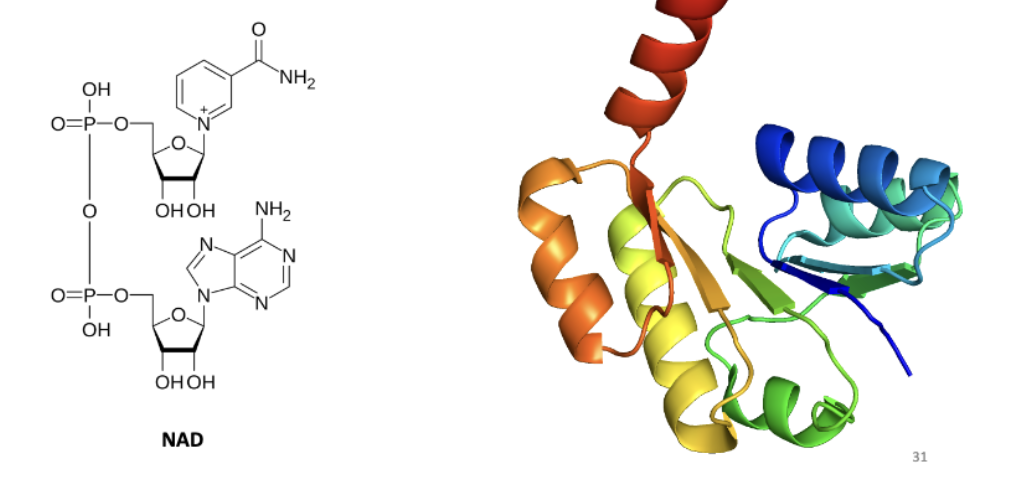
Nicotinamide adenine dinucleotide (NAD) binding domain
Binds important redox transfer cofactor used by many enzyme
What is RNase?
(ribonuclease): digestive enzyme secreted from pancreas
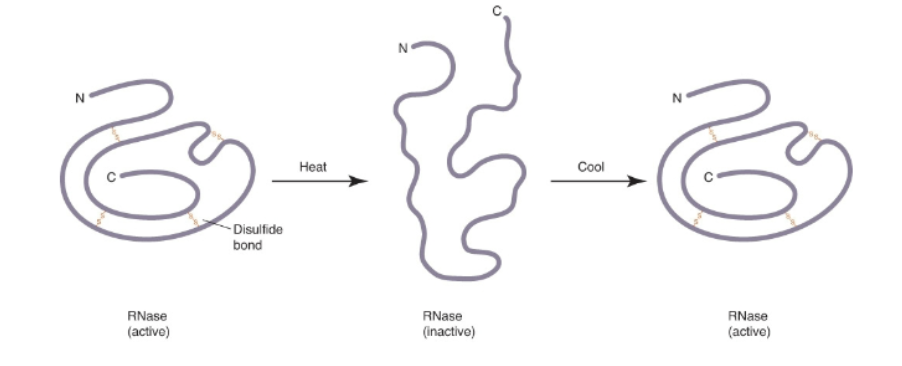
What was RNase used for?
Used to study enzyme properties
1959: Christian Anfinsen used RNase to assert that primary structure determines higher levels of structure
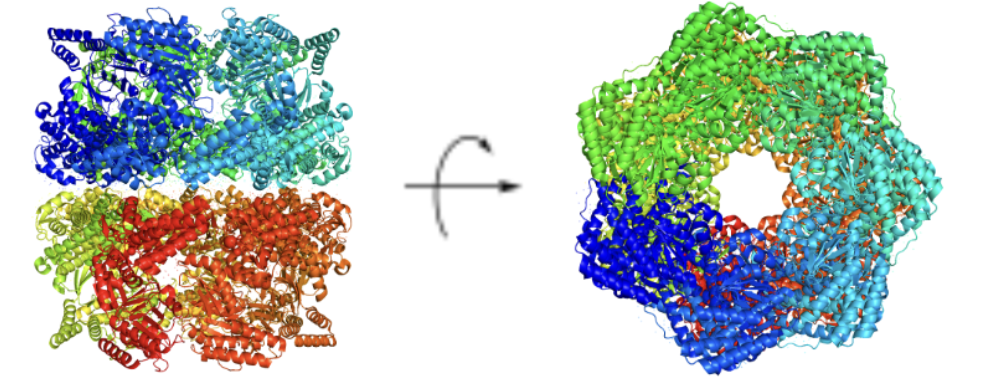
GroEL
Primarily found in bacteria (E. coli)
• Aids in protein folding for about ~15% cytosolic E. coli proteins
• Belongs to the Group I chaperonine family
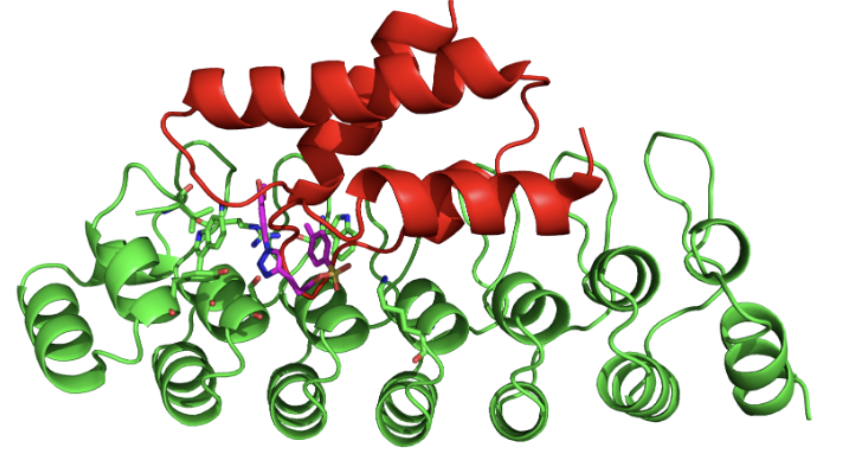
Green = gankyrin; Magenta = cjoc42; Red = S6 ATPase
Oxygen Binding in Myoglobin and Hemoglobin
Oxygen-binding proteins in mammals (reversible binding)
o Indirect binding by oxygen to heme iron
Myoglobin
single chain (MW=17,000 Da)
Hemoglobin
four monomer units (each=17,000 Da)
o 2α and 2β subunits
o Four heme molecules bind each hemoglobin tetramer
• Bound to protein to assist its function (prosthetic group)
Prosthetic group
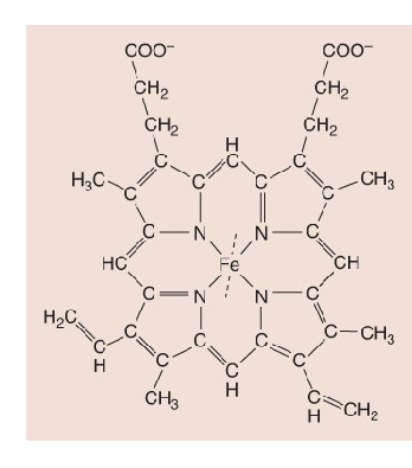
The Fe (iron); the purpose is to catalyze and function; nonpeptide
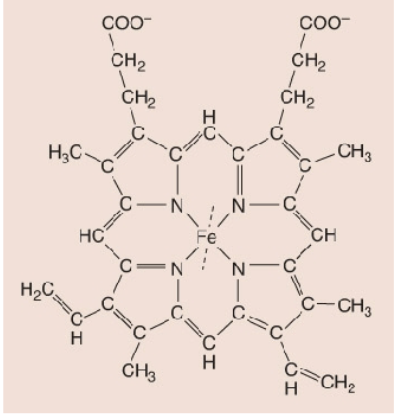
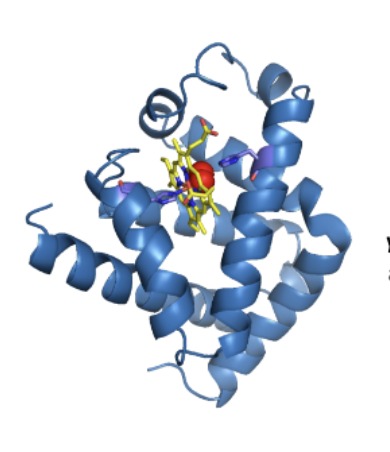
Yellow= prothetic group (Fe2+)
Red= oxygen
What is a Heme?
4 linked pyrrole rings, each chelated to Fe2+
fractional saturation
Y = [MbO2]/([Mb] + [MbO2])
What is MB (myoglobin)?
Mb stores oxygen in muscle cells and releases oxygen when needed
Hemoglobin: quaternary structure (4 monomer subunits)
Each monomer has a bound heme group which can bind oxygen
Influence binding of a subsequent molecule of oxygen
What is cooperativity?
Influence binding of a subsequent molecule of oxygen
What is non-cooperative?
independent monomer binding
What is positive cooperativity?
Increase in binding affinity occurs with partially bound hemoglobin
Graph of Behavior of oxygen binding to hemoglobin
o Y is the fractional saturation
o S-shape indicates positive cooperativity
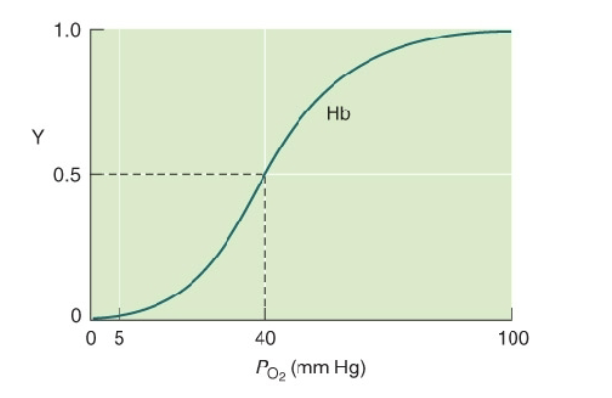
Behavior of oxygen binding to hemoglobin
O2 binds Mb more avidly than Hb
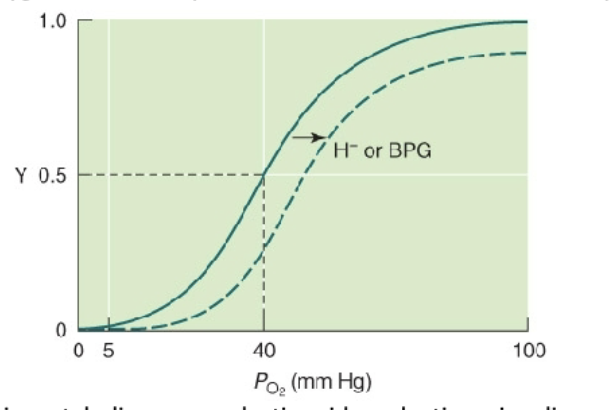
What is the Bohr Effect?
Diminished binding of oxygen to Hb in response to increase in the concentration of protons.
Explanation: Greater level of anaerobic metabolism causes lactic acid production, signaling weaker binding of O2 to Hb, leading to its release
Protein Purification
o Must first extract the protein and keep its properties intact
o Need a method to measure the protein
• Measure disappearance of substrate or appearance of product (assay)
o Major concern is denaturation and is minimized in several ways
• Perform experiments in a cold room
• Use of buffers to prevent extreme pH changes
• Addition of reagents to prevent oxidation of Cys (i.e., DTT, TCEP, etc.)
• Use of protease inhibitors to prevent peptide bond hydrolysis
o Extraction process may use physical methods to release intracellular contents
o Once we have the protein in solution, we need to fractionate
• Separation of proteins by differences in solubility, size, charge, and affinity.
What is the main purification method?
chromatography
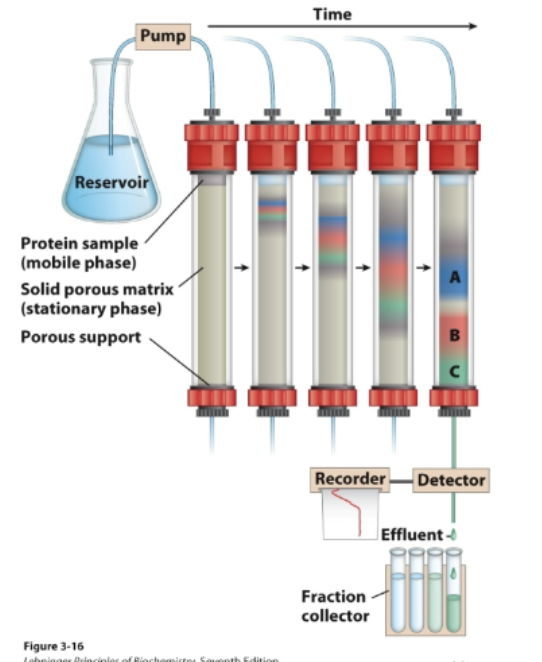
Chromatography
Separation of components due to their different
affinities towards two distinct phases: a mobile phase
and a stationary phase
• Proteins with a lower affinity for the solid phase will
wash off first; proteins with higher affinity will retain on
the column longer and wash off later.
Types of Chromatography
Ion exchange column chromatography; Size exclusion chromatography; Affinity chromatography
Ion exchange column chromatography is a common method
Rely on the electrostatic interaction between protein and the stationary phase
Size exclusion chromatography
Large proteins emerge first
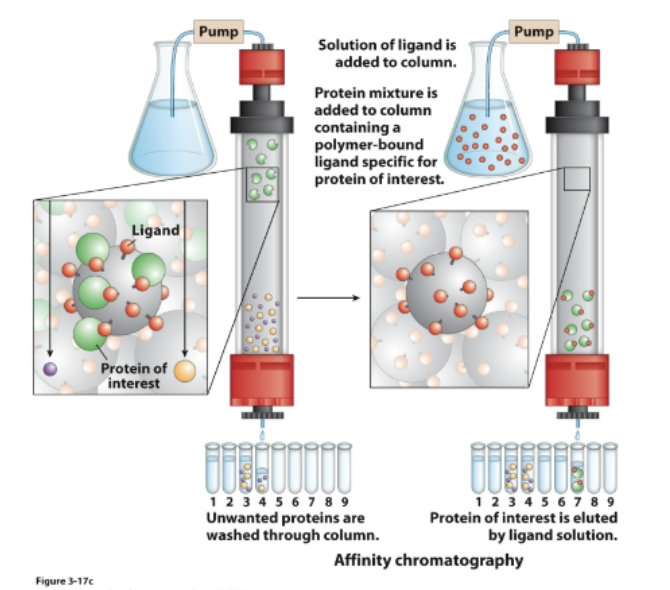
Affinity chromatography
• Protein binds a ligand immobilized on the stationary phase
• Highly specific type of chromatography
• Protein of interest is retained in the stationary phase
• Then the protein of interest is eluted by passing a solution of free ligand
Assays
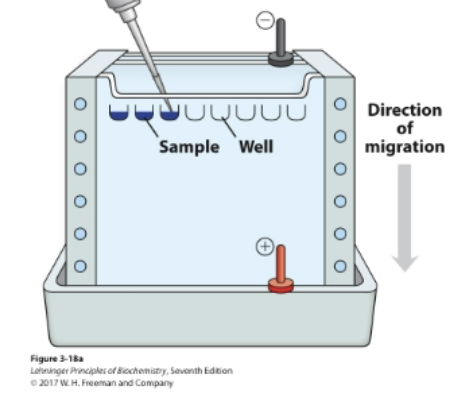
Separation in analytical scale is commonly done by electrophoresis.
What do Assays do?
The electric field pulls proteins according to their charge.
• The gel matrix hinders the mobility of proteins according to their
size and shape.
SDS- sodium dodecyl sulfate
A detergent
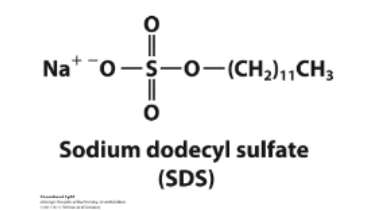
What do SDS micelles do?
bind to proteins and facilitate unfolding.
• SDS gives all proteins a uniformly negative charge.
• The native shape of proteins does not matter.
• The rate of movement will only depend on size: small proteins will move faster
UV-Visible spectroscopy
The aromatic amino acids absorb light in the UV region.
For proteins and peptides with known extinction coefficients (or sequences), concentration can be determined by UV-visible spectrophotometry using the Lambert-Beer law: A =ebc
Mass spectrometry
Molecules are fragmented, vaporized, and separated.
Mass spectrometer detects charged fragments and peaks report the mass divided by the charge (m/z) of the detected species
X-Ray Crystallography
Requires high-quality crystals of the proteins of interest (high purity, regular geometry)
Elucidates the molecular structure of small and large compounds
Can elucidate the structures of proteins bound to other molecules, such as natural substrates or drugs