VSEPR Geometries
0.0(0)
Card Sorting
1/5
Earn XP
Description and Tags
Last updated 3:24 AM on 9/23/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
1
New cards
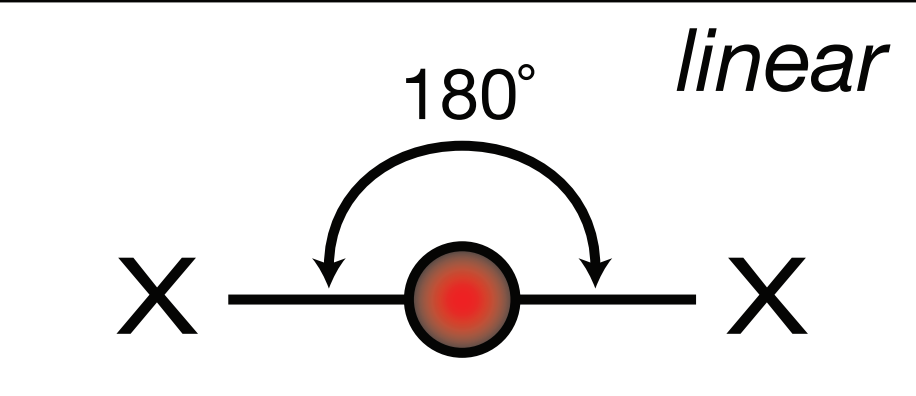
Steric Number: 2, Lone Pair(s): 0
Electron Geometry: Linear, VSEPR Geometry: Linear, Bond Angle: 180o
2
New cards
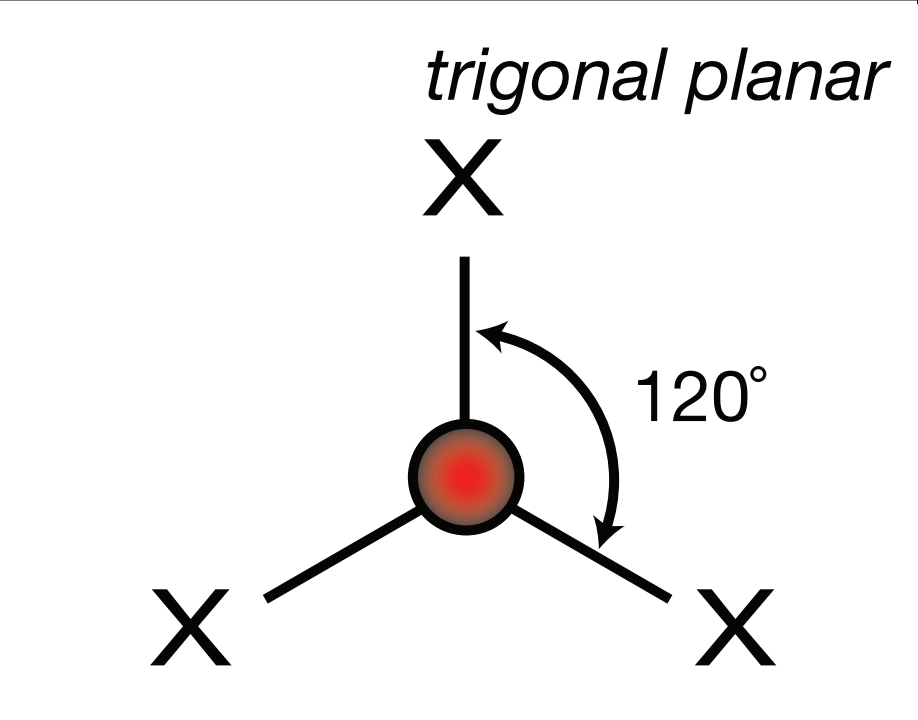
Steric Number: 3, Lone Pair(s): 0
Electron Geometry: Trigonal Planar, VSEPR Geometry: Trigonal Planar, Bond Angle: 120o
3
New cards
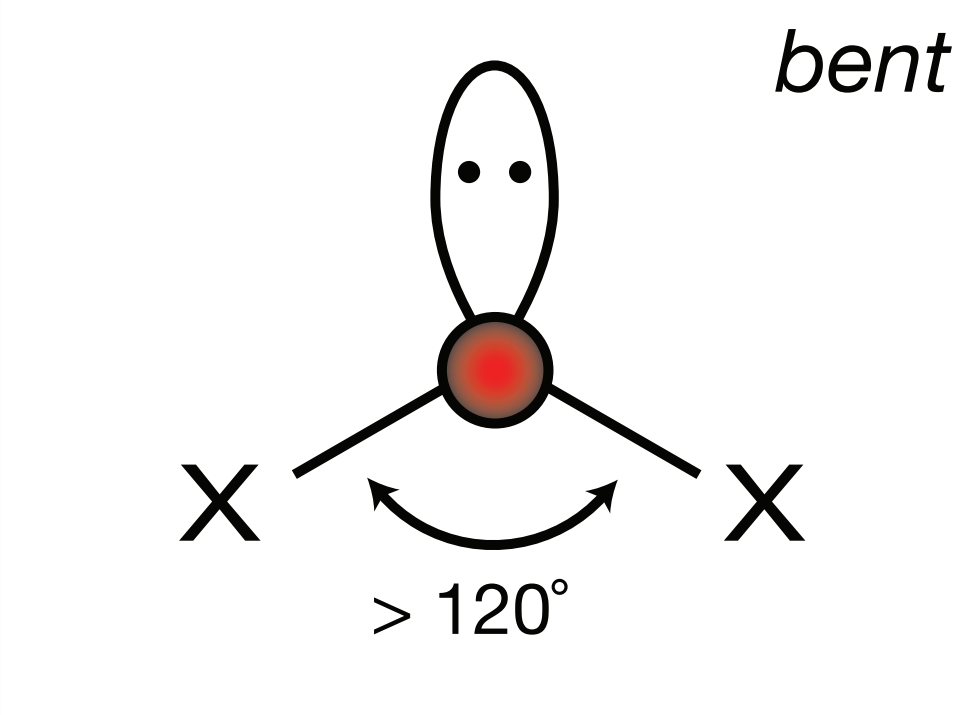
Steric Number: 3, Lone Pair(s): 1
Electron Geometry: Trigonal Planar, VSEPR Geometry: Bent, Bond Angle: <120o
4
New cards
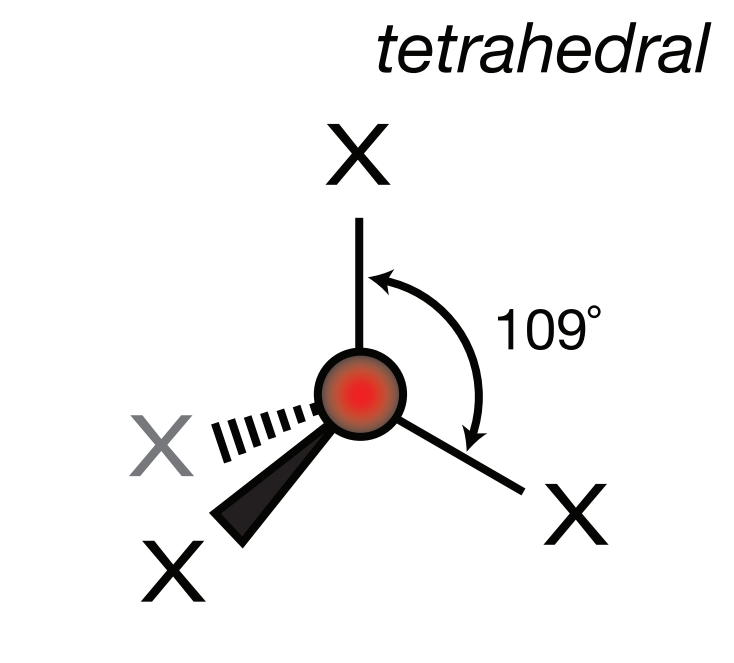
Steric Number: 4, Lone Pair(s): 0
Electron Geometry: Tetrahedral, VSEPR Geometry: Tetrahedral, Bond Angle: 109.5o
5
New cards
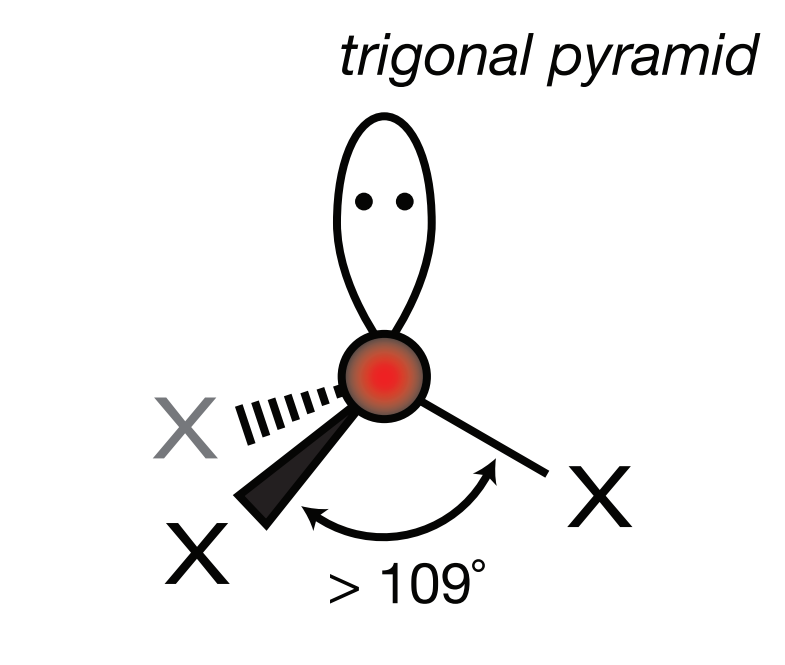
Steric Number: 4, Lone Pair(s): 1
Electron Geometry: Tetrahedral, VSEPR Geometry: Trigonal Pyramid, Bond Angle: 107o
6
New cards
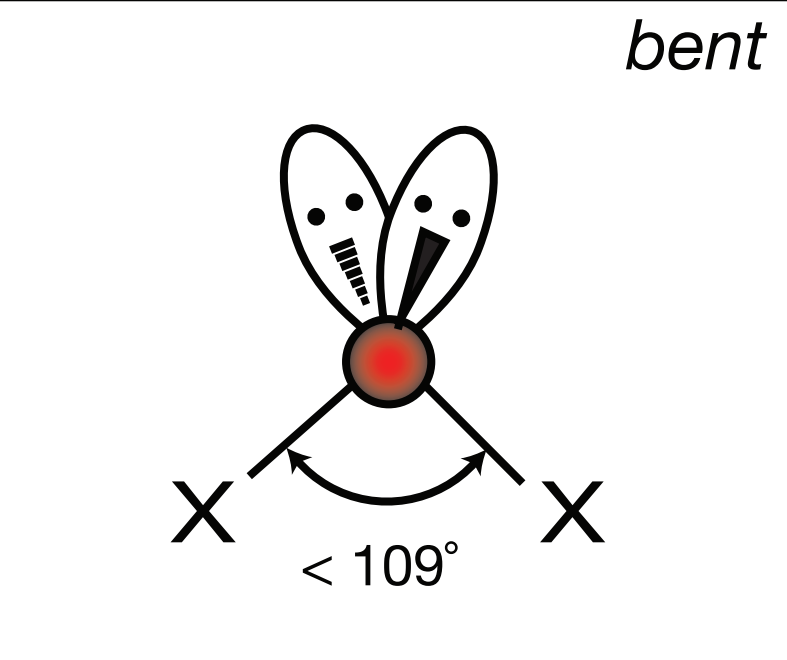
Steric Number: 4, Lone Pair(s): 2
Electron Geometry: Tetrahedral, VSEPR Geometry: Bent, Bond Angle: 104.5o