Atoms and Foundation of Chemistry - Chapter 1 & 2 Review
1/15
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Relative Atomic Mass
Symbol is Aᵣ
Average weighted mass of the atoms of an element compared with 1/12 of the mass of a carbon-12 atom.
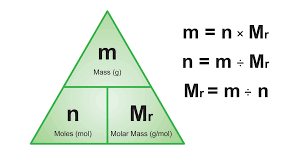
Mole
Avogadro’s Number (6.0×10²³ /mol)
Number of specified entities as there are atoms in exactly 12.0g of Carbon-12.
Relative Formula Mass
The sum of the relative atomic masses of the atoms in the formula unit of an ionic compound.
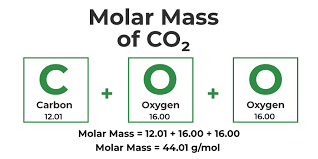
Molar Mass
Mass of 1 mol of a substance.
Equal to it’s relative molecular or formula mass in grams.
Limiting and Excess Reagent
Limiting reagent: completely used up first in a reaction, thus determining the maximum amount of product formed. Determines maximum yield.
Excess reagent: remains after the limiting reagent is used up.
Types of Yields (3)
Theoretical Yield: Calculated maximum amount of product that could form form a given amount of reactants.
Actual Yield: Amount of product actually obtained from a reaction.
Percent Yield: Percentage of Actual over Theoretical.
Percent Composition by Mass
Percentage by mass of each element present in a compound.
Empirical Formula
Simplest whole-number ratio of atoms each element in a compound.
Molecular Formula
Actual number of atoms of each element present in a molecule.
Ions
A charged particle formed when an atom or compound loses or gains electrons.
Anions are negative. Cations are positive.
Spectator Ions
Ions that do not react or change state in a reaction.
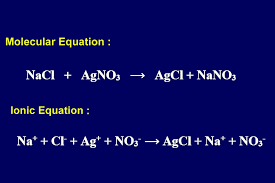
Solution
A homogenous mixture of a solute dissolved in a solvent.
Concentration
Symbol is C
The amount of solute per unit volume of a solution.
Mol/dm³
Molarity
A way of expressing concentration.
Mol/dm³
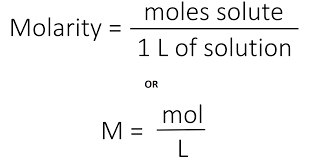
Molar Gas Volume
Symbol is Vm
The volume occupied by 1 mol of gas at a certain pressure and temperature.
At RTP: 24.0 dm³/mol
At STP: 22.4 dm³/mol
RTP and STP
Room Temperature and Pressure (25 Celsius, 298 Kelvin, 1.01×10^5 Pascals)
Standard Temperature and Pressure (0 Celsius, 273 Kelvin, 1.01×10^5 Pascals)
1 atm for both.