Cancer Chemotherapeutic Drugs 1: Alkylating Agents
1/27
Earn XP
Description and Tags
1. Describe the mechanism of action of nitrogen mustards. 2. Differentiate the structure-activity relationship of the nitrogen mustards. 3. Compare and contrast the activation and toxicities of cyclophosphamide and ifosfamide. 4. Describe the mechanism of action of mesna as a chemoprotective agent.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
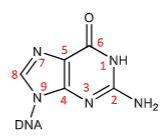
N-7 of guanine is the target of Nitrogen mustards (alkylating agents) that crosslink DNA leading to inhibition DNA replication causing cell death.
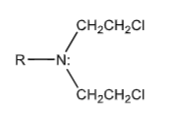
Nitrogen mustard’s general structure
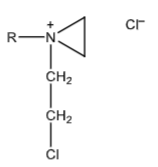
Aziridinium ion intermediate electrophile that attacks N-7 of guanine. The aziridinium intermediate is formed by an intramolecular nucleophilic attack of the unionized amine on the β-carbon of the mustard.
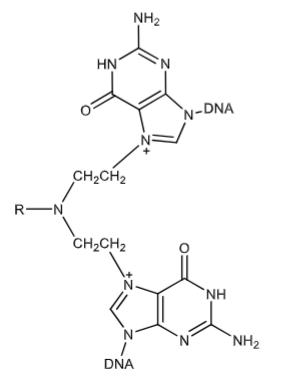
Nitrogen mustard that has fully crosslinked DNA via alkylation with covalent bonds. This interferes with DNA replication and transcription of DNA to RNA leading to death of cancerous cells. Can produce intra and inter strand crosslinks and DNA-protein crosslinks.
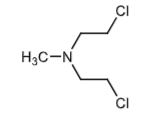
Mechlorethamine (Valchlor topical gel): alkylating agent, antineoplastic agent. Tx: mycosis fungoides (cutaneous T-cell lymphoma). Too reactive to be administered orally b/c it makes the aziridinium ion too quickly before it gets to the site of action.
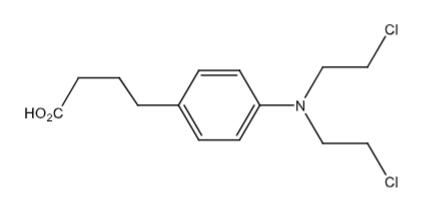
Chlorambucil (Leukeran oral tablet): alkylating agent, antineoplastic agent. Tx: Chronic lymphocytic leukemia, Hodgkin lymphoma, etc. Arylamine decreases reactivity to allow for oral administration.
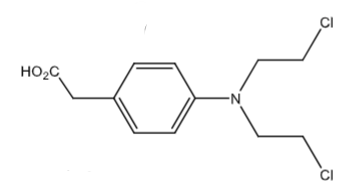
Phenylacetic acid mustard: weakly active metabolite of chlorambucil via beta-oxidation.
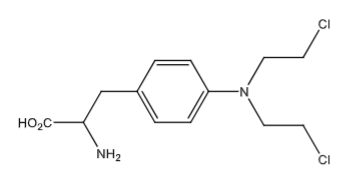
Melphalan (Generic oral tab and soln for inj; Evomela soln for inj): alkylating agent and antineoplastic agent Tx: multiple myeloma. Aromatic ring decreases nucleophilic nature of the nitrogen to decrease reactivity allowing PO admin. Phenylalanine uses L-type amino-acid transporter-1 (LAT1) to get into tumor cells (which is overexpressed in multiple myeloma)
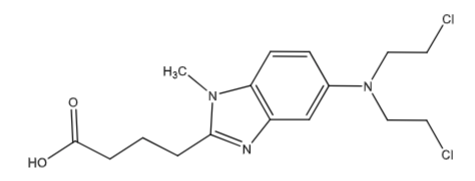
Bendamustine (Belrapzo, Bendeka soln for inj): Alkylating agent and antineoplastic agent Tx: chronic lymphocytic leukemia and multiple myeloma. MOA: “not fully understood” causes miotic catastrophe at various cell cycle stages and stimulates p21 and p53 tumor suppressor proteins leading to induced apoptosis
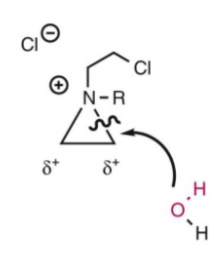
Aqueous decomposition of nitrogen mustards. H2O attacks the aziridinium ion twice to an inactive dehalogenated diol. Avoid by increasing the stability of the nitrogen mustard in solution. Buffer the solution to make it acidic. This makes the H2O less nucleophilic b/c it will be protonated and makes the nitrogen less nucleophilic.
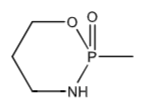
Oxazaphosphorine ring base structure for Oxazaphosphorine alkylating agents (a type of nitrogen mustard)
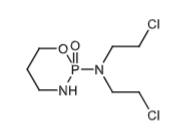
Cyclophosphamide (PO & IV): Alkylating agent with phosphamide group that decreases reactivity allowing it be activated in the cells.
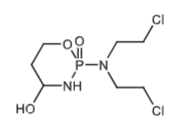
Metabolite of cyclophosphamide via CYP2B6 and 3A4. This is transported into the cells.
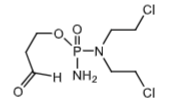
Alsophosphamide (metabolite of cyclophosphamide via a reversible non-enzymatic mechanism). This is transported into the cells.
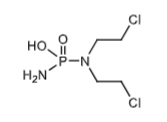
Phosphoramide mustard of cyclophosphamide via beta-elimination from aldophosphamide. Ionized at physiologic pH which traps this molecule in the cell to form the aziridinium ions which will crosslink the DNA.
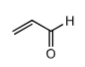
Acrolein: toxic metabolite of cyclophosphamide and ifosfamide when the mustard is formed. This causes hemorrhagic cystitis leading to bladder toxicity (urotoxic) and hematuria. It is critical patients drink plenty of water while on cyclophosphamide to minimize urotoxicity caused by acrolein. Ifosfamide has significantly greater urotoxicity compared to cyclophosphamide b/c higher doses are needed and it is more water soluble so it concentrates in the renal system causing more damage.

Ifosfamide (IV only): nitrogen mustard alkylating agent. Actively secreted into the renal system. Enters proximal convoluted tubule via organic cation transporter protein-2 (OCT2) Cyclophosphamide does not*
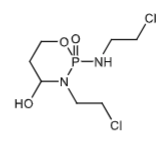
4-hydroxyifosfamide is a metabolite of Ifosfamide via CYP3A4 and 2B6.
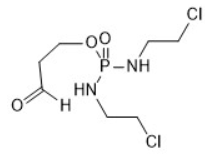
Aldofosfamide formed in vivo from ifosfamide. Aldophosphamide is in equilibrium with 4-hydroxyifosfamide.
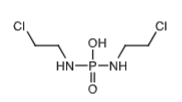
Ifosfamide mustard that is ionized and trapped in cells to form aziridinium ion to cross link DNA
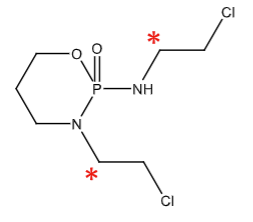
Hydroxylation sites of ifosfamide leading to dechloroethylation creating a toxic metabolite (chloroacetaldehyde). 50% of ifosfamide dose undergoes dechloroethylation to the toxic metabolite.

Hydroxylation sites of cyclophosphamide leading to dechloroethylation creating a toxic metabolite (chloroacetaldehyde). 10% of cyclophosphamide dose undergoes dechloroethylation to the toxic metabolite. Less toxic
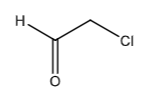
Chloroacetaldehyde: toxic metabolite of ifosfamide and cyclophosphamide formed in a process called dechloroethylation. ADRs: Neurotoxicity (seziures, coma, mental status changes) and nephrotoxicity (intrinsic injury)
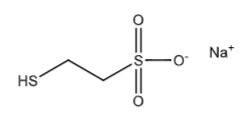
Mesna (mercaptoethane sulfonate sodium, Mesnex): Chemoprotectant, minimizes hemorrhagic cystitis caused by ifosfamide and cyclophosphamide. Mesna is highly polar, ionized, and highly water soluble which makes it concentrate in the urine. It binds acrolein to form an inactive adduct. Mesna also stabilized 4-hydroxyphosphamide so acrolein cannot be formed in the bladder to begin with. Mesna only protects against hemorrhagic cystitis not chloroacetaldehyde induced toxicities.

Mesna and acrolein to inactive adduct that is excreted in the urine
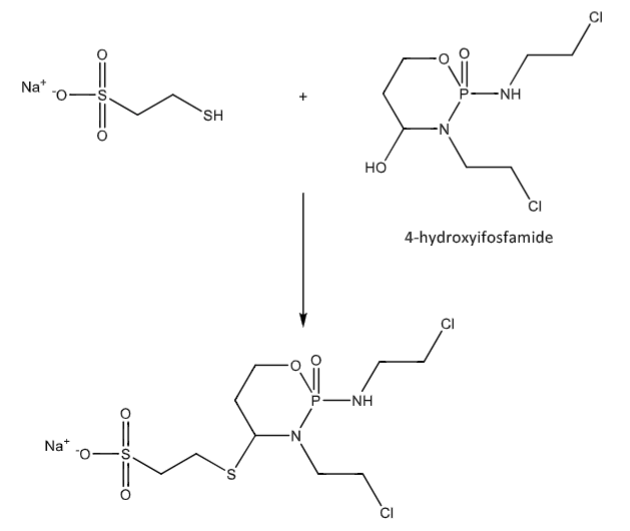
Mesna and 4-hydroxyifosfamide to inactive adduct that is excreted in the urine. Acrolein is unable to form.

Why does not MESNA interfere with antineoplastic efficacy of ifosfamide?
“doesn’t get into tissue” Mesna stays in the vascular compartment b/c its highly polar and ionized. Cannot interfere with the cellular level’s action of ifos. or cyclophos.
In circulating blood mesna undergoes rapid oxidation to dimesna which is inactive. Then dimesna is freely filtered at the kidneys. In the kidneys, 1/3 of dismesna undergoes the following:
dimesna and glutathione via thiol transferase forms mesna (active) and a glutathione-mesna adduct
Glutathione-mesna adduct and glutathione via thiol transferase forms mesna (active) and a glutathione-glutathione adduct
glutathione-glutathione adduct and NADPH and H+ via glutathione transferase forms 2 glutathiones (free) and NADP+
Overall, mesna inactivates acrolein formation before it gets to the bladder via regional detoxification.
Alkylating agents: nitrogen mustards characteristics and adverse effects
Cell cycle-phase nonspecific neoplastic agents: more toxic to cells in late GI or S phase of cell cycle (DNA is unwinding and exposing nucleotides)
Not selective to cancer cells
They target rapidly diving cells (normal and cancerous):
Bone marrow: myelosuppression (leukopenia (decreased WBCs), thrombocytopenia (platelets), anemia)
GI tract: stomatitis (inflammation of mouth and lips)/ oral ulcers (N/V/D)
Hair follicles: alopecia/hair loss
Monoalkylated products are related to genotoxicity (mutagenicity):
Risk of secondary malignancy induced by nitrogen mustard. Tx of initial cancer but secondary malignancy caused later from chemotx.