IB Chem: Bonding & Structure
1/39
Earn XP
Description and Tags
Ionic Model, Covalent Model, Metallic Model & From Models to Materials
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Lattice Dissociation Enthalpy
the standard enthalpy change that occurs on the formation of 1 mole of gaseous ions form the solid lattice
endothermic process
Cations
positively charged ions
Ions
An electrically charged atom formed by the loss or gain of electrons
Anion
negatively charged ion
Cation
positively charged ion
Ionic Bond
strong electrostatic force of attraction between oppositely charged ions
Giant Ionic
high melting and boiling points
not volatile
Volatility: the vaporization of a chemical
Soluble — can form ion-dipole bonds
only conduct electricity when molten or in solution
hard, brittle
electrostatic attraction between ions
at room temp - solid
e.g. NaCl
Giant Metallic
moderately high melting/boiling point
electrical conductivity only when solid or liquid
insoluble
hard, malleable
solid — at room temp
delocalised electrons attracting positive ions
e.g. copper
Simple Covalent
low melting/boiling point
does not conduct electricity
usually insoluble unless polar
soft
at room temp - solid, liquid or gas
weak, intermolecular forces and covalent bonds within a molecule
e.g. Br2
Giant Covalent
very high melting/boiling point
does not conduct electricity
(except graphite)
insoluble
very hard(diamond and silicone) or soft(graphite)
electrons in covalent bonds between atoms
e.g. graphite, silicone(IV), oxide
Metallic Bonding
the structure of metallic bonding has positive metal ions suspended in a “sea” of delocalised electrons
very strong electrostatic forces between the positive metal centres
Delocalised Electrons
free moving electrons not bound to their atom
Malleability
to be malleable —> when a force is applied, the metal layers can slide
e.g. metals can be hammered into sheets
attractive forces between the metal ions and electrons act in all directions
Strength
metallic compounds are strong and hard due to the strong attractive forces between the metal ions and delocalised electrons
Electrical Conductivity
can conduct electricity in the solid/liquid state
due to mobile electrons that freely move around and conduct electricity
outer electrons increase across a period, # of delocalised charges increases
Na = 1 outer electron
Mg = 2 outer electrons
Al = 3 outer electrons
ability to conduct electricity increases across a period
Ductile
the ability for a metal to be stretched or drawn into a thin wire without breaking
Thermal Conductivity
metals are good thermal conductors due to the behaviour of their cations and delocalised electrons
cations in metal lattic vibrate vigorously as their thermal energy increases
delocalised electrons carry increased kinetic energy and transfer it rapidly throughout the metal —contributes to high thermal conductivity
Melting & Boiling Point
metals have high melting/boiling points
due to strong electrostatic forces of attraction between the cations and delocalised electrons in the metallic lattice
Alloy
a mixture of two or more metals or nonmetals — to produce a substance with metallic properties
mixed physically but not chemically combined
ions of the different metals are spread throughout the lattice and are bound together by the delocalized electrons
Uses of metals
Aluminum is used in food cans because it is non-toxic and resistant to corrosion and acidic food stuffs
copper is used in electrical wiring b/c it is a good electrical conductor and malleable/ductile
stainless steel is used for cutlery as it is strong and resistant to corrosion
Melting point trends
melting point of metal increases moving across a period, from left to right
greater chard difference leads to a stronger electrostatic attraction, and therefore a stronger metallic bond
Ionization Energy
The amount of energy required to remove an electron from an isolated atom/molecule
Electron Affinity
how readily a neutral atom(in gaseous state) will attract and hold onto an extra electron, forming a negative ion
the measure of an atom’s “attractiveness” for an electron
Electronegativity
the ability of an atom to draw an electron pair towards itself in a covalent bond
Trends in Melting Points of Metals
strength of electrostatic attraction can be increased by:
increasing the number of electrons
increasing the number of positive charges
decreasing the size of the cations(ionic radius)
Brass
substitutional alloy
copper & zinc
strong & resistant to corrosion
e.g door handles, hinges
Steel
interstitial alloy
iron, carbon and other elements
very strong
e.g. construction, bridges, cars
Stainless steel
iron, chromium, nickel & carbon
corrosion resistant
cutlery, surgical instruments, cookware
Solder
lead and tin
low melting point
e.g., joining metals in electrical circuits metals and jewelry
Bronze
copper tin
hard and strong resistance to corrosion
medals & sculptures
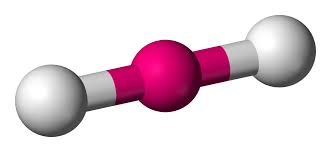
Linear
e.g. BeCl2, CO2 & ethyne
angle = 180 degrees
two electron domains
0 lone pairs
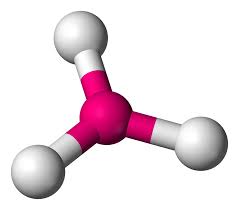
Triangular Planar/Trigonal Planar
3 electron domains
0 lone pairs
120 degrees
e.g. BF3, CH2O
boron trifluoride, ethene and methanal
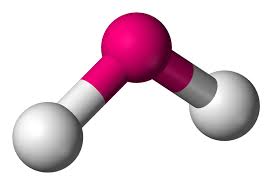
Molecular Geometry: Bent Linear
Domain geometry: Trigonal Planar
e.g. SO2(sulfure dioxide)
118 degrees
1 lone pair
‘expands the octet’
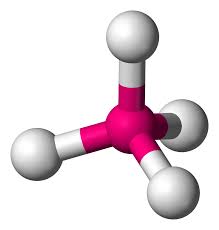
Tetrahedral
109.5 degrees
0 lone pairs
e.g. methane & ammonium(CH4 & NH4+)
Molecular Geometry: Trigonal Pyramidal
Domain Geometry: Tetrahedral
107 degrees
1 lone pair
e.g. ammonia(NH3)
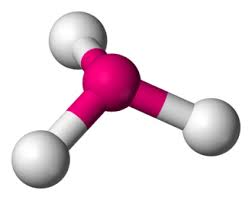
Molecular Geometry: Bent/Angular Linear
Domain Geometry: Tetrahedral
104.5 degrees
2 lone pairs
e.g. water
Van der Waals’ forces
a term used to include:
london dispersion forces
dipole- induced dipole attractions
dipole-dipole attractions
forces occur between molecular(intermolecularly) as well as within moelcule(intramolecularly)
London dispersion forces
instantaneous induced dipole - induced dipole forces that exist between all atoms and molecules
caused by temporary dipoles
constantly appearing and disappearing due to constant motion of electrons
weakest bond
Strength depends:
the number of electrons in the molecule
surface area of the molecules
Hydrogen Bonding
strongest type of intermolecular force
special type of permanent dipole - permanent dipole bonding
Trigonal Bypyramidal
composed of a central atom and five surrounding atomms
Bond angles: 90, 120, 180
e.g. PCl5
Seesaw
3-D
central atom has one lone pair & 4 bonding pairs
Bond angles < 180
90 - 120
e.g. SF4
VSPER Theory
electron pairs(both bonding and non-bonding) repel each other and arrange themselves to minimize repulsion