Lecture 14: Synaptic Transmission and Vesicle Cycle
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
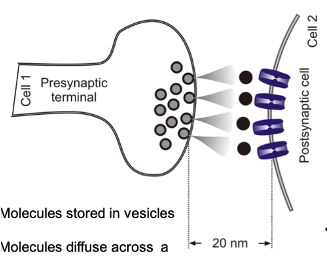
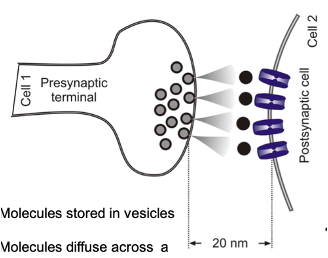
Chemical Synapses
Molecules stored in vesicles are released from one cell onto another to produce an effect
Molecules diffuse across a “gap” between cell membranes
~20nm distance
Signalling: relatively slow (~0.5msec)
Puts a ‘break’ on one cells ability to affect another
Unidirectional
Post-synaptic cell may release a retrograde transmitter which will affect the pre-synaptic cell
Majority of synaptic transmission in the nervous system occurs via these synapses
Electrical Synapses
Small regions of adjacent membranes come into close contact
Gap junctions present at the site of contacts to bridge the membranes of both cells to allow the movement of ions and small molecules (~500M.W)
Holes” in adjoining cell membranes: linked by channels - gap junctions or connexons (hexameric - made up of connexin subunits)
Signalling: very fast - near instantaneous electrical signalling between cells, what occurs in one cell will happen in another cell due to the transfer of ions
Bidirectional
Important for direct electrical coupling between cells - electrical synchronization e.g. in the heart
Relatively rare in the nervous system – occurs in the inhibitory interneurons or local networks e.g. neocortex, retina
Function of Chemical Synapses
They allow for flexibility and modification of nervous system function through
Neural computation: integration of many input +/- to generate, process, neural signals to produce an output
Exhibit plasticity: supports development, learning and memory by chaining the strength of neural connections involved in the organisation and encoding of information
Drug targets: affect neurotransmitter synthesis, release, receptors, uptake, and degradation.
Functional Flexibility: Produce complex effects by modifying neural activity.
6 Criteria For Neurotransmitter Classification
Synthesised and stored in the pre-synaptic neurone (vesicles)
Released upon stimulation of that neurone in a depolarisation and Ca2+-dependent manner
Located in the appropriate region at levels sufficient to evoke physiological responses
Compound must reproduce physiological effects when applied to a postsynaptic neurone
Transmitter recognition & signal transduction mechanisms (receptors etc.) associated with that postsynaptic neurone
Transmitter removal mechanisms
Small Molecule Neurotransmitters
A class of neurotransmitters that includes:
Amino acids e.g. Glycine, GABA, glutamate
Amine e.g. Noradrenaline, Dopamine and Serotonin
Purine e.g . Adenosine and ATP
Peptide Neurotransmitters
A class of neurotransmitters that consists of short-chained polypeptide sequences and include:
Endorphins/ Enkephalins/ Dynorphin
Neuropeptide Y
Somatostatin
Substance P
vasoactive intestinal Peptide etc
Dales Principle
The idea that a neuron releases only one type of neurotransmitter at all of its synapses
It’s been challenged by the co-existence and release of small molecule neurotransmitters and peptides by interneurons, such as GABA and enkephalins in the striatum, and the release of more than one neurotransmitter in some projection pathways, such as L-glutamate and dopamine in the VTA to nucleus accumbens pathway.
Significance of VTA-Nucleus Accumbens Pathway
It was thought to be solely dopaminergic, but it is actually both dopaminergic and glutamatergic
Small Synaptic Vesicles (SSVs)
‘Clearer synaptic vesicle’ – contains small molecule NT
Cell type: Neurone
Diameter: 40nm
Location: synapse active zones
Active zone – pre-synaptic release site opposite the post-synaptic density
VGCa2+ Channels: Located close by – part of the active zone
Contents: Small neurotransmitter
Threshold concentration - [Ca2+] 200uM
High concentration threshold explains the close proximity of the channels – a high threshold to initiate the release process
Physiology: Single Action Potential
Biogenesis: Constitutive local vesicle, recycling transmitter synthesis and uptake
Large Dense Cored Vesicles (LDCVs)
Cell type: Neurons and neuroendocrine cells
Diameter: ~100nm
Location: non-specific, diffuse i.e all over the cell
Originated from the ER and Golgi
Transported via the microtubule system and axon transport system to potential release sites
Not specifically associated with the active zone
distributed relatively diffusely in pre-synaptic terminals
VGCa2+ Channels: Located relatively distant
Contents: Peptides, and proteins (sometimes noradrenaline)
[Ca2+]: 5-10uM – more sensitive to changes in intracellular Ca2+ due to the distance from the channel – to initiate release of more diffuse elevation of [Ca2+]I from the VG Ca2+
Physiology: Involved in repetitive AP activity
Biogenesis: Highly regulated ER-derived vesicles (no recycling) transmitter production and processing under direct genomic control; trafficked to release sites
Classical Vesicle Cycle
Exocytosis:
Vesicles associate with the plasma membrane and release their contents.
The vesicle membrane fuses with the plasma membrane and adds to it.
Endocytosis:
Recover membrane added during exocytosis.
Vesicles formed from the endosome act as a "holding tank" for membrane and neurotransmitter (NT) storage.
Reserve Pool:
A ‘backup’ pool of vesicles that are ready to be mobilized and fused with the membrane for NT release.
New vesicles filled with NT from the endosome enter the reserve pool,
Visualised with electron microscopy
Docking and Priming
Vesicles under a 2 stage process where they become attached and primed to the membrane ready to undergo function
Heavily ATP-dependent process
Electron Microscopy Visualisation of Reserve Pool
Synapse with pre-synaptic ending - large .o vesicles associated
Tissue treated with Antibodies for synapsin
Depletion of some of the vesicles - distinction of the reserve pool – disruption of synapsin action allows the vesicles to disperse and disappear
Vesicles that remain are actively associated with the membrane – can’t dissociate
Slam Freezing
Technique developed by Heuser and Reese → provided evidence for full vesicle fusion and collapse
Involved rapidly cooling frog neuromuscular junction on a metal block after electrical stimulation of motor neurone axon fibres to initiate acetylcholine release
Freeze fracture electron microscopy is used to visualise the presynaptic membrane
Sections of the presynaptic membrane were visualised at different times after stimulation to follow any changes in presynaptic membrane structure:
Pits formed at 4ms and persisted after 8ms, indicating sites of vesicle fusion
Presynaptic Vesicles:
40-50 nm in diameter, clear centres, spherical.
Contains thousands of neurotransmitter molecules based on volume.
Problem: Increased membrane surface area due to fusion.
Solution: Vesicle recycling maintains membrane area homeostasis.
Vesicle Cycle: Step 1 - Docking
The close association with plasma membrane
Don’t dissociate if the plasma membrane is treated with an Ab
Synaptic vesicles only dock at the active zone
This is the presynaptic area adjacent to the signal transduction machinery of the postsynaptic membrane
Active zones differ between neurones (by vesicle number) - reflects their function and the functions they support
Vesicle Cycle: Step 2 - Priming
Prepares vesicles for release
Synaptic vesicle “maturation” process
Vesicles made into a competent state to release transmitter (in response to Ca2+)
Requires ATP w/o won’t occur
May be required for a conformational change in proteins that drive the release
If this step doesn’t occur, the vesicles won’t release
Vesicle Cycle: Step 3 - Fusion/ Exocytosis
The release of vesicle contents
Full fusion of synaptic vesicle and presynaptic terminal membrane
Requires Ca2+ (increased)
Involves Ca2+ “sensor” protein – detects and initiates release
Fusion induces exocytosis - contents discharged (diffusion) – full fusion and vesicle membrane becomes part of the cell
Takes about 1msec
Results in the continual addition of membrane to the plasma membrane
Vesicle Cycle: Step 4 - Endocytosis
The recovery and recycling of vesicle fused membrane via endocytosis
Vescular membrane recovery is mediated by certain proteins associated with the membrane that will assist in its recovery
It is triggered by increased intracellular Ca2+, membrane is recovered as clathrin associates with the protein on the membrane used to form the vesicle to form a clathrin coated pit
In the presence of ATP, it will trigger the release of the vesicle from the plasma membrane to be recycled back
Involves cytoskeletal protein lattice formation (from clathrin monomers) to help pinch off the membrane
Takes about 5 secs and is ATP-dependent
Spontaneous pit formation occurs following the vesicular relase
Vesicle Cycle: Step 5: Recycline
The mechanism used to conserve synaptic vesicle membrane via endosome
Trafficked back to the endosome to remove clathrin
Sits in the endosome in a reserve buffer
Vesicles refill with transmitter (ATP-dependent again - concentrating neurotransmitter)
The entire vesicle cycle (docking to refilling) takes about 1 minute – QUICK/ RAPID TURNOVER as the process is biochemically driven
Kiss and Run
An alternative vesicle fusion model
It suggests that full vesicle fusion may not be required for neurotransmitter release
When the vesicle is activated to release its contents, fusion pores form
These are discreet openings between the membrane and the vesicle, which allow neurotransmitters to leak out (down a concentration)
SSVs are recycled intact from the cell membrane and are not recycled as clathrin-coated vesicles via the endoscope
Vesicle structure is maintained
Kiss and Run Model: Electron Microscopy Evidence
Full vesicle collapse was observed in some areas of the membrane.
Vescilce integrity is mainated and the formation of narrow conduits/ pores that allow the passage of neurotransmitters are seen
This model is harder to confirm with EM since it only provides snapshots; functional studies are more definitive.
Evidence of Kiss and Run Model - Functional Studies
Measure activity during NT release following an increase in intracellular [Ca²⁺], which triggers membrane depolarisation and VG Ca²⁺ channel activation.
Membrane capacitance can be recorded
Increased capacitance indicates increased membrane surface area during fusion.
Full fusion shows a step-up in capacitance (more membrane added).
In kiss and run, capacitance briefly increases and returns to baseline, suggesting a transient increase in surface area (flickering capacitance).
Vesicular Fusion: Capacitance and Surface Area
Membrane capacitance is proportional to surface area.
In the full fusion model, membrane capacitance steadily increases, whereas in kiss and run, the change is brief and reversible
Kiss and Run Mechanism of Vesicular Fusion/Release
Fast recycling
Low capacity - only a few vesicles over time in the active zone
Favoured at low frequency stimulation
70-80% of glutamate release in the hippocampus mediated via this
Classical Mechanism of Vesciular Fusion/Release
Slow recycling
High capacity - many vesicles over time
Favoured at high frequency stimulation
Release Machinery Proteins
Proteins that associate with vesicles or the active zone of the plasma membrane
They ensure vesicular engagement with the cell membrane and the initiation of the release proteins
Involves VAMPs - vesicle assocaited membrane proteins
Vesicle Associated Proteins
Synapsins
Synaptobrevins
Synaptoatagmins
Rab proteins effectors
Synaptophyins
SV2s, SCAMPs, CSPs
Neurtransmitters transporter
Vacuolar proton pump
Amphipysins, AP2 clathrin
CaMKI and CamKII, pp60src
Dyamin 1
Dynein, kinesin
Plasma Associated Proteins
SNAP-25
Syntaxins
Voltage Gated Ca2+
Complexins
Munc18s – important in ensuring priming of vesilces
NSF and SNAPs – important in allowinging vesciles to disengage from the membrane
Muc13s
Nerexins
SNARE Proteins
Proteins that become entangled to allow vesicles to engage in the membrane
There are 2 types
v-SNARE e.g synaptobrevin
t-SNARE, e.g. SNAP-25
Synaptobrevin
A v-SNARE located on the vesicle (VAMP)
It is 18kDa
Single transmembrane spanning
Syntaxin
A t-SNARE located on the target membrane
35kDa
Single transmembrane spanning
Has a regulatory domain important for priming vesicles
SNAP-25 (Synaptosomal-associated protein 25)
A t-SNARE located on the target membrane
25kDa
Anchored to membrane by S-acylation
S-Palmitolyation
Post-translational modification that allows the attachment of proteins to the plasma membrane and the cytosolic/ inner leaflet
Docking of Vesicle (Release Machinery)
Involves Synaptobrevin (on vesicle) and SNAP-25 and Syntaxin (on target membrane)
Synaptobrevin, Syntaxin, and SNAP-25 form a 1:1:1 trimeric complex, known as the SNARE complex.
This complex is a coiled-coil structure of α-helices.
SNAP-25 has two α-helical regions
The α-helices of the SNARE proteins wrap tightly around each other, bringing the vesicle into close contact with the target membrane.
This interaction ensures the vesicle is docked and ready for fusion.
how vesicles move from the reserve pool to associate with the cytoskeleton
Priming of Vesicles (Release Machinery)
The SNAREs form a tighter complex → believed to store energy to facilitate the release process
SNARE pins form via zippering, where the coiled-coil complex between the 3 SNARE proteins becomes tighter - orchestrated by the regulatory domains of syntaxin
SNARE pins trigger vesicular fusion in response to a signal
Process in ATP dependent - uses MunC18 (ATPase)
Synpatotagmin
A 65kDa Ca2+ sensor found on vesicles
It has a cytosolic region where Ca2+ are able to bind and cause a conformation change which then initiates the fusion process
In the absence of Ca2+ it binds to SNARE pins in priming
In the presence of Ca2+, it binds to phospholipids and causes the VAMP to vesicle into the membrane for fusion
Release Machinery in Vesicular Fusion
They are activated through a conformational change in synaptotagmin
It alters the configuration of the release machinery that pulls the vesicle closer to the membrane and releases the energy stored in the SNARE pin (and SNARE complex) to pull the membranes apart and allow fusion to occurr
NSF
An ATPase that facilitates the dissociation of SNAREs.
SNAREs are tightly coupled in coiled-coil structures that keep the vesicular membrane fused with the plasma membrane.
It uses ATP to "uncoil" the SNAREs, allowing them to dissociate.
This dissociation is crucial for:
Internalisation of empty vesicles.
Re-docking of another vesicle with the same T-SNAREs.
It binds to the SNARE-pin complex to facilitate SNARE release.