IUPAC system
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
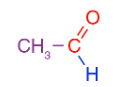
IUPAC name of CH3CHO is
A - Ethanal
B - Ethanol
C - Acetaldehyde
D - Acetone
A
IUPAC name of CH3-O-C2H5
A - Methoxy ethane
B - Ethoxy ethane
C - Methyl ethane
D - Ethyl ethane
A
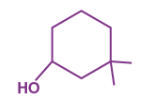
IUPAC name for this structure
A → 3,3 -dimethyl - 3 - oxocylohexane
B → 1,1 -dimethyl - 3 - cyclohexanol
C → 3,3- dimethyl - 1 - cyclo-oxanol
D → 1,1 -dimethyl - 1 - cyclo-oxanol
B
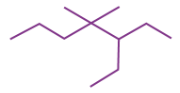
IUPAC name for this structure
A → 4-ethyl-3, 3-dimethylheptane
B → 4-ethyl-4, 3-dimethylheptane
C → 3-ethyl-3, 4-dimethylheptane
D → 3-ethyl-4, 4-dimethylheptane
D
IUPAC name of acetylsalicylic acid
A → 2-acetoxy benzoic acid
B → 1-acetoxy benzoic acid
C → 4-acetoxy benzoic acid
D → 3-acetoxy benzoic acid
A
Dienes are the name given to compounds with ________
A → One double bond
B → Two double bonds
C → More than one double bond
D → None of the above
B
Identify the smallest alkane which can form a ring structure:
A → Ethane
B → Cyclo ethane
C → Propane
D → Cyclopropane
D
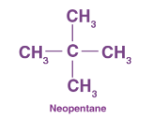
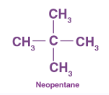
IUPAC name for neopentane:
A → 3, 3–dimethylpropane
B → 1, 2–dimethylpropane
C → 2, 3–dimethylpropane
D → 2, 2–dimethylpropane
D
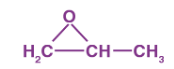
What is the name for the following compound:
A → 1, 2-epoxy propane
B → 2, 2-epoxy propane
C → Epoxy propane
D → None of the above
A
Identify the formula of ethanol:
A → CH3CHO
B → CH3COCH3
C → CH3CH2CHO
D → CH3CH2OH
D
What is the order of priority of functional groups?
Carboxylic Acid > Sulfonic Acid > Esters > Acid Halides > Amides > Cyanides > Aldehyde > Ketones > Alcohols > Amines > Alkynes > Alkenes > Alkanes
What is a functional group?
A functional group is the group of atoms in a molecule that determines the chemical behaviour of the molecule.
It determines the chemical properties of the molecule.
Give examples of a few functional groups along with their formula.
Carboxylic Acid ( -COOH)
Alcohol ( -OH)
Aldehyde ( -CHO)
Ketone (-CO-)
Ester ( -COOR)
Cyanide ( -CN)
Amide ( -CONH2)
Acid Halide ( -COOCl)
Sulfonic Acid ( -SO3H)
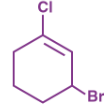
IUPAC name for this compound:
A → 3-Bromo-1-chlorocyclohexene
B → 1-Bromo-3-chlorocyclohexene
C → 3-Bromo-1-chlorohexidine
D → 1-Bromo-3-chlorohexidine
A
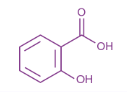
Structure of salicylic acid
Structure of neopentane
O-xylene
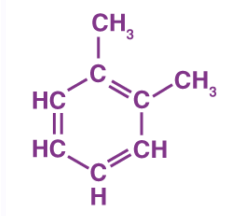
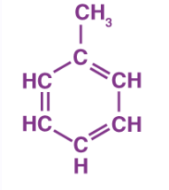
Toluene
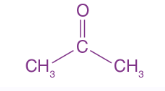
Acetone
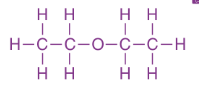
Diethylether
How many C atoms in CH4
(Methane)
1
How many C atoms in C2H6
(Ethane)
2
How many C atoms in C3H8
(Propane)
3
How many C atoms in C atoms in C4H10
(Butane)
4
How many C atoms in C5H12
(Pentane)
5
How many C atoms in C6H14
(Hexane)
6
How many C atoms in C7H16
(Heptane)
7
How many C atoms in C8H18
(Octane)
8
How many C atoms in C9H20
(Nonane)
9
How many C atoms in C10H22
(Decane)
10