Chemistry - Halogen Displacement Reactions
0.0(0)
0.0(0)
Card Sorting
1/10
Earn XP
Description and Tags
Edexcel IGCSE Chemistry
Last updated 11:38 AM on 12/16/24
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
1
New cards
what is a halogen displacement reaction?
a more reactive halogen displaces a less reaction halogen from a solution of it’s salts
2
New cards
what is the equation for a displacement reaction between chlorine and potassium iodide?
* chlorine + potassium iodide → potassium chloride + iodine
* Cl2 + 2KI → 2KCl + I2
* Cl2 + 2KI → 2KCl + I2
3
New cards
why does chlorine displace iodine?
beacuse chlorine is more reactive
4
New cards
name the group 7 elements in order from most reactive to least reactive
1. fluorine
2. chlorine
3. bromine
4. iodine
5. (astatine)
5
New cards
what happens to the electron shells of group 7 elements when they take part in a reaction?
the outershell gains an eletron and forms an anion
6
New cards
the less easily these anions form …
… the less reactive the elements is
7
New cards
explain the reactivity trend of the halogens?
* atoms become larger down the group
* the outershell moves further away from the nucleus
* force of attraction between the nucleus and outershell decreases
* outer electron is gained less easily
* halogen becomes less reactive
* the outershell moves further away from the nucleus
* force of attraction between the nucleus and outershell decreases
* outer electron is gained less easily
* halogen becomes less reactive
8
New cards
what is a redox reaction?
where oxidation and reduction happen at the same time
9
New cards
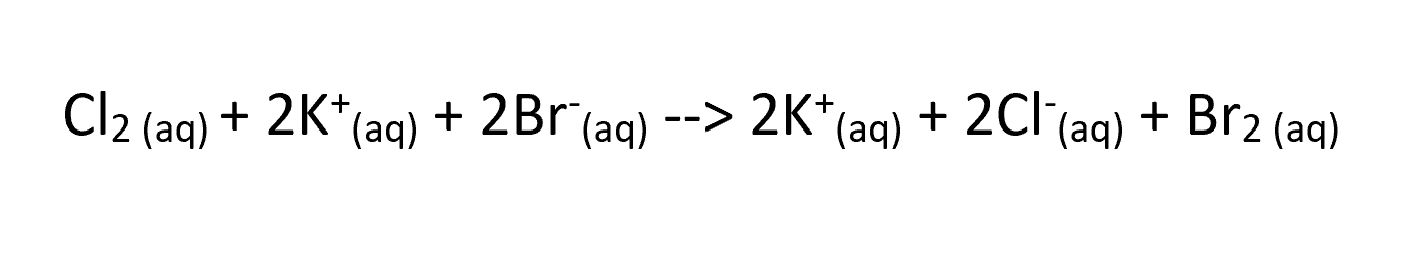
in this equation which ion is the spectator ion?
K+
10
New cards
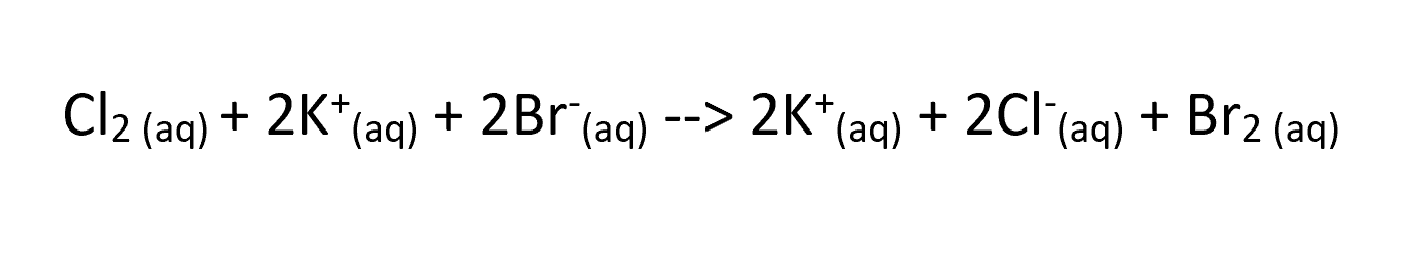
in the equation what ion is being reduced?
Chlorine
11
New cards
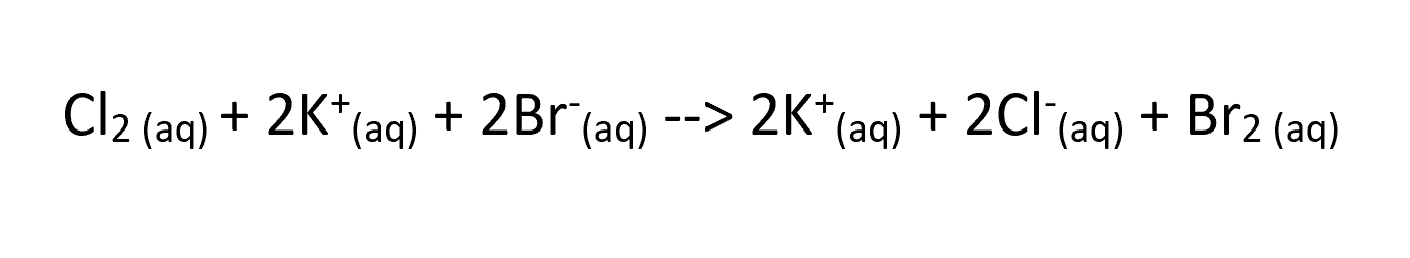
in the equation what ion is being oxidised?
Bromine