UWG Principles of Biology Chapters 2-3
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
“Elements of Life”
Oxygen, Carbon, Hydrogen, Nitrogen
Elements’ properties depend on
Atoms
Atoms are made of prots/neuts/elects, which subatomic particles compose the nucleus?
Protons, Neutrons
Atomic number
Number of protons
Chemical behavior of atom depends mostly on electron no. in
valence shell (outermost)
Mass number is..
Twice the atomic number; sum of protons & neutrons
Chemical bonds
Attractive force linking 2 or more atoms together
Covalent bonds
Type of chem. bond where a pair of valence electrons are shared by two atoms filling their valence shell
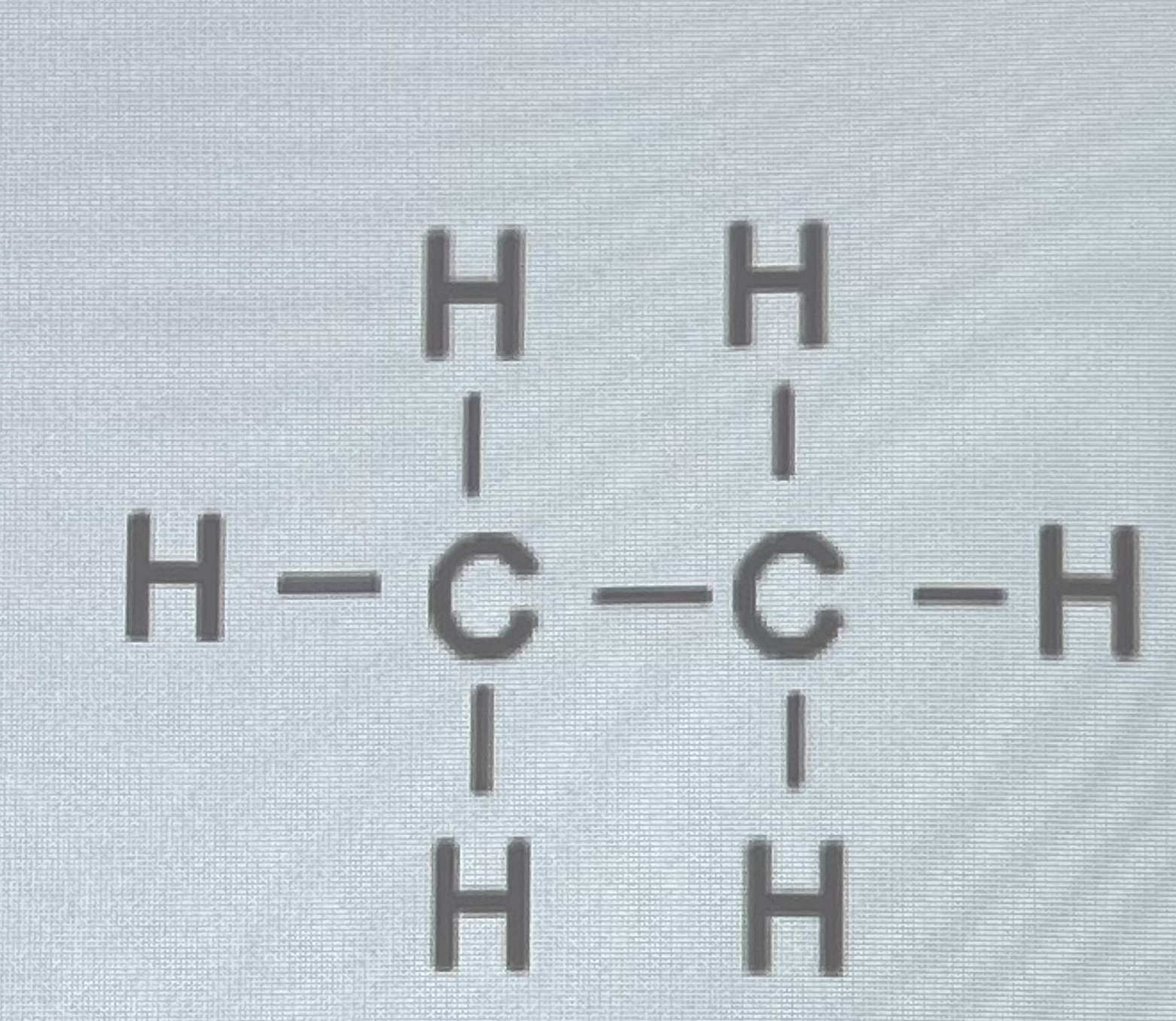
What’s this bond?
Single bond - 1 pair of electrons shared
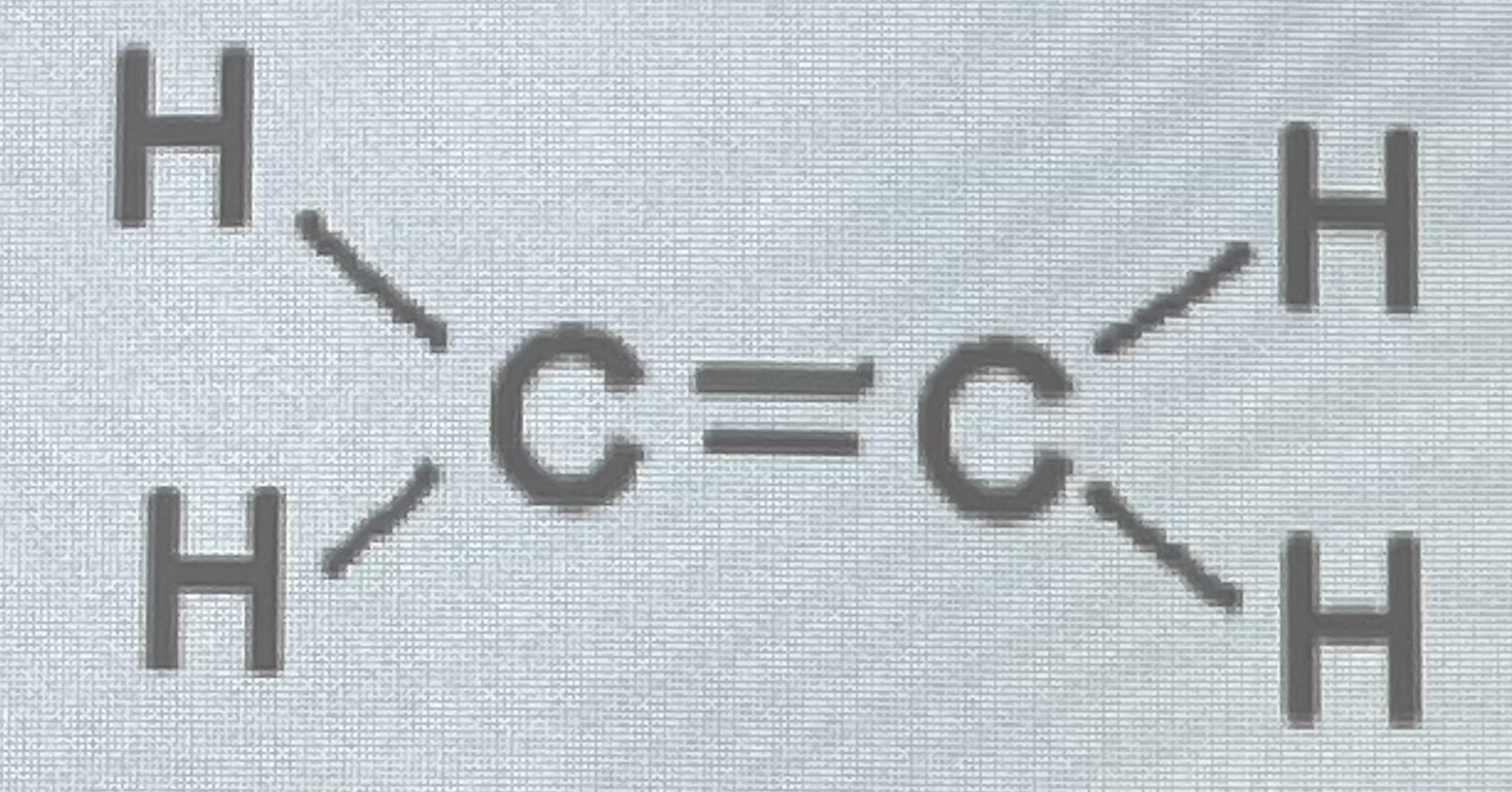
What’s this bond?
Double bond - 2 pairs of electrons shared

What’s this bond?
Triple bond - 3 pairs of electrons shared
Unequal sharing of electrons occurs within molecules due to
electronegativity
Nonpolar covalent bond
Atoms share electrons equally
Polar covalent bond
One atom more electroneg; unequal sharing
Ions
Form when atoms gain or lose 1 or more electron
Cations
positively charged ions
Anions
negatively charged ions
Hydrogen bonds
Bond between partial positive & partial negative charges on hydrogen atom of water molecule
Cohesion
Water molecules clinging to each other (via hyd bonds)
Adhesion
Water molecules clinging to external substances
Heat is ___ when hydrogen bonds break
Absorbed
Heat is ___ when hydrogen bonds form
absorbed
High Specific Heat
Amt. of heat energy required to raise temperature of 1g substance by 1 Celsius