Chemistry: Polymers
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
What are polymers?
large molecules built by joining hundreds smaller molecules called monomers to form long chains.
What are plastics?
synthetic polymer
What is a monomer?
A single subunit which when joined together form a polymer together.
What is addition polymerization?
Formation of polymer by chain addition reactions between monomers that contain an unsaturated bond
many monomers are added together
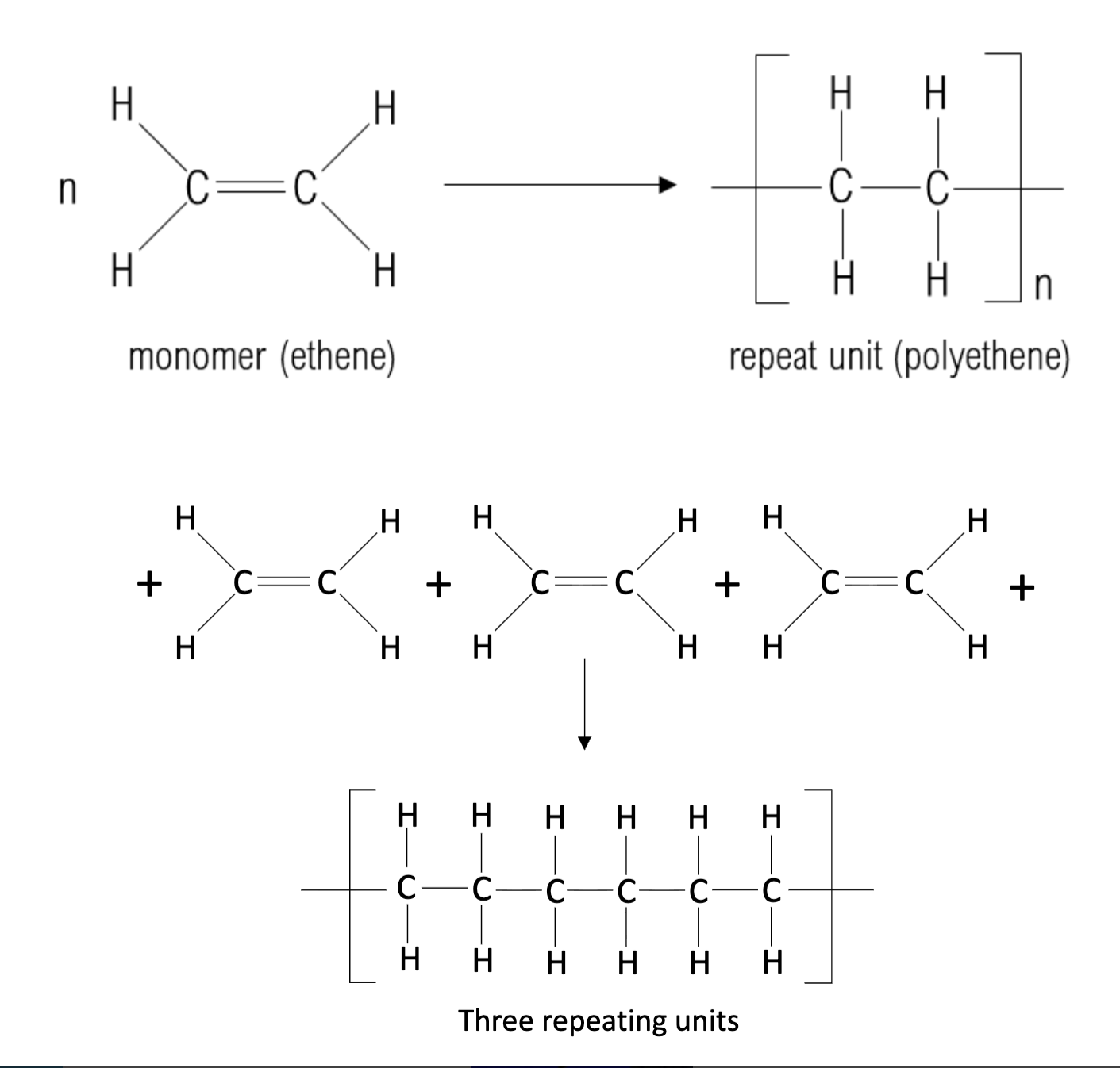
How do you draw addition polymerization?
1. Draw two C atoms that were in
the double bond with a single
covalent bond.
2. Draw the brackets and the ‘n’.
3. Add the links outside the brackets.
4. Add the atoms /groups that were
attached to each C atom of the double
bond.
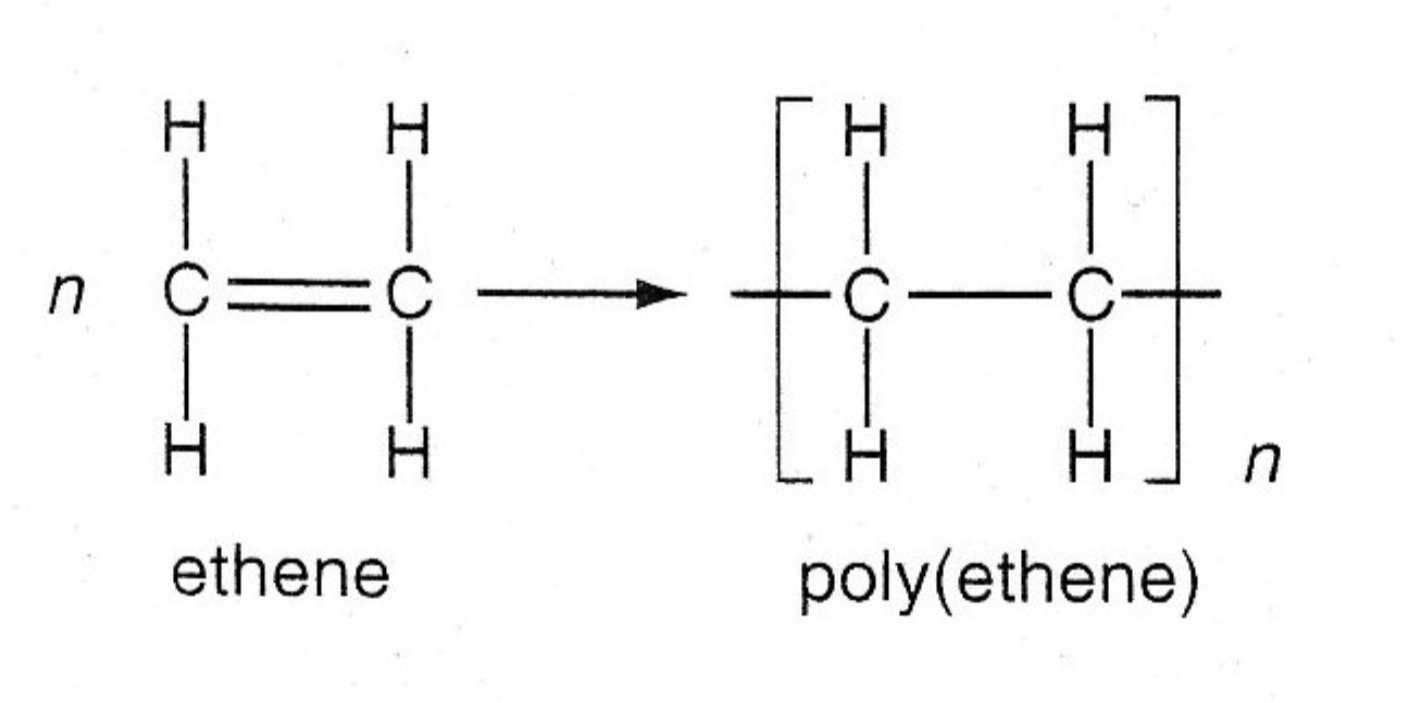
What are the issues with synthetic polymers?
Non-biodegradable and produce toxic gases when burnt.
What does biodegradable mean?
A substance that can be naturally broken down by micro-organisms
What are biodegradable plastics?
Plastics designed to be broken down (decomposed) by bacteria