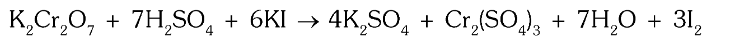Potassium Dichromate
1/41
Earn XP
Description and Tags
Part 2 of the series (d- and f- block). Question mode: Flashcards only. Answer mode: Answer with Definition. Suitable for JEE Mains, I don't know about JEE Advanced. Catered towards NCERT and IAT.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
42 Terms
What (ore) is Potassium Dichromate from?
chromite or chrome iron
FeO\cdot Cr_2O_3 or FeCr_2O_4
What are the three basic steps of preparation of K_2Cr_2O_7?
Conversion of chromite into sodium chromate
Conversion of sodium chromate into sodium dichromate
Conversion of sodium dichromate into potassium dichromate
How is chromite converted to sodium chromate?
It is obtained by the fusion of chromite ore with sodium hydroxide or sodium carbonate in the presence of air (O_2).
What is the chemical reaction for conversion of chromite to sodium chromate using sodium carbonate?
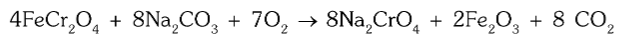
What is the chemical reaction for conversion of chromite to sodium chromate using sodium hydroxide?

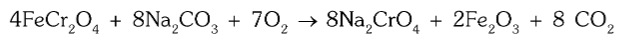
How is sodium chromate separated from ferric oxide?
by filtration
How is sodium chromate converted to sodium dichromate?
by acidification with sulphuric acid
What is the chemical reaction for conversion of sodium chromate to sodium dichromate?
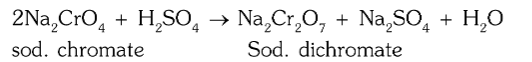
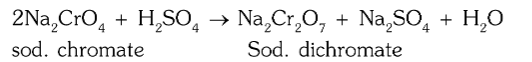
How is sodium dichromate separated from sodium sulphate?
sodium sulphate is less soluble and crystalized out first on cooling the mixture
What is the colour of sodium dichromate?
orange
What is the colour of potassium dichromate?
orange
How is sodium dichromate converted to potassium dichromate?
It is treated with potassium chloride
What is the chemical reaction for conversion of sodium dichromate to potassium dichromate?
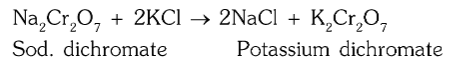
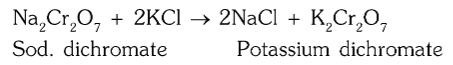
How is potassium dichromate separated from sodium chloride?
orange potassium dichromate crystals are crystallized out of the solution or whatever
What is the chemical reaction when you heat K_2Cr_2O_7?
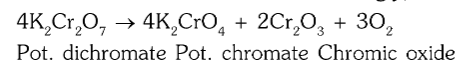
What gas is evolved on the heating of potassium dichromate?
Oxygen
Cr_2O_7^{-2} converts to CrO_4^{-2} in ________ medium
(acidic, basic)
basic
CrO_4^{-2} converts to Cr_2O_7^{-2} in ________ medium
(acidic, basic)
acidic
Whta is the colour of Cr_2O_7^{-2}?
orange
What is the colour of CrO_4^{-2}?
yellow
What colour change do you observe when treating K_2Cr_2O_7 with an alkali?
orange turns to yellow
What is the chemical reaction for treating potassium dichromate with potassium hydroxide?
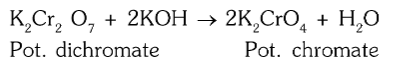
What happens when you acidify K_2CrO_4?
It changes back to K_2Cr_2O_7.
What is the melting point of potassium dichromate?
670 K
What is the chromyl chloride test?
potassium dichromate gives orange/red vapouds of chromyl chloride on heating with concentrated sulphuric acid and metal chlorides
What is the chemical reaction for the chromyl chloride test?
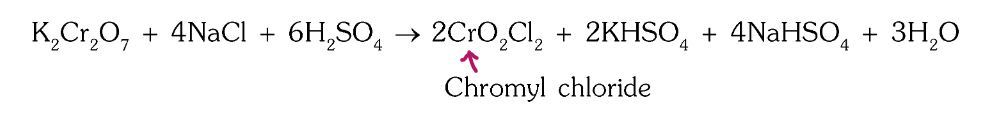
What is the chemical formula of chromyl chloride?
CrO_2Cl_2
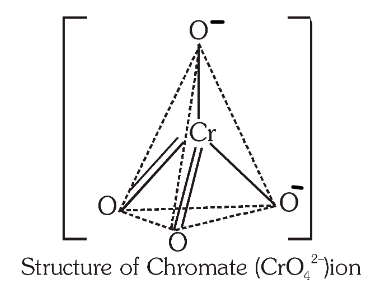
What is the structure of the chromate ion?
What is the structure of the dichromate ion?
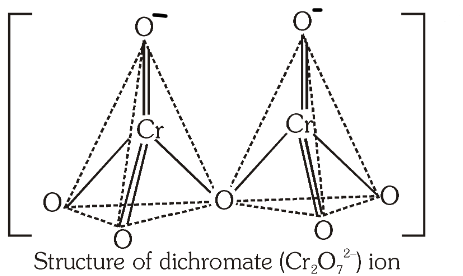
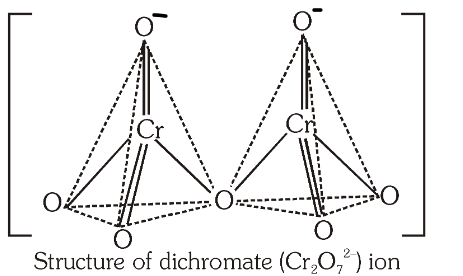
What is the bond angle between the Cr-O-Cr bond?
126 degrees
What is the chemical equation for reaction of potassium dichromate with hydrogen peroxide?
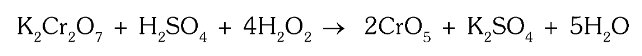
What is the ionic chemical reaction that depits the oxidising nature of dichromates?
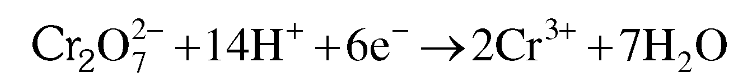
dichromate can oxidise 2I^- to?
I_2
dichromate can oxidise 2Cl^- to?
Cl_2
dichromate can oxidise Fe^{+2} to?
Fe^{+3}
dichromate can oxidise Sn^{+2} to?
Sn^{+4}
dichromate can oxidise SO_2 to?
SO_4^{-2}
dichromate can oxidise H_2S to?
S
dichromate can oxidise NO_2^- to?
NO_3^-
What is the oxidation state of Cr_2O_7 in potassium dichromate?
-2
What is the oxidation state of Cr in potassium dichromate?
+6
What is the chemical equation for the reaction of potassium dichromate with potassium iodide?