ATI TEAS 7: Acids, Bases, and pH Key Concepts
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Acid
Substance that releases hydrogen ions (H⁺) in solution
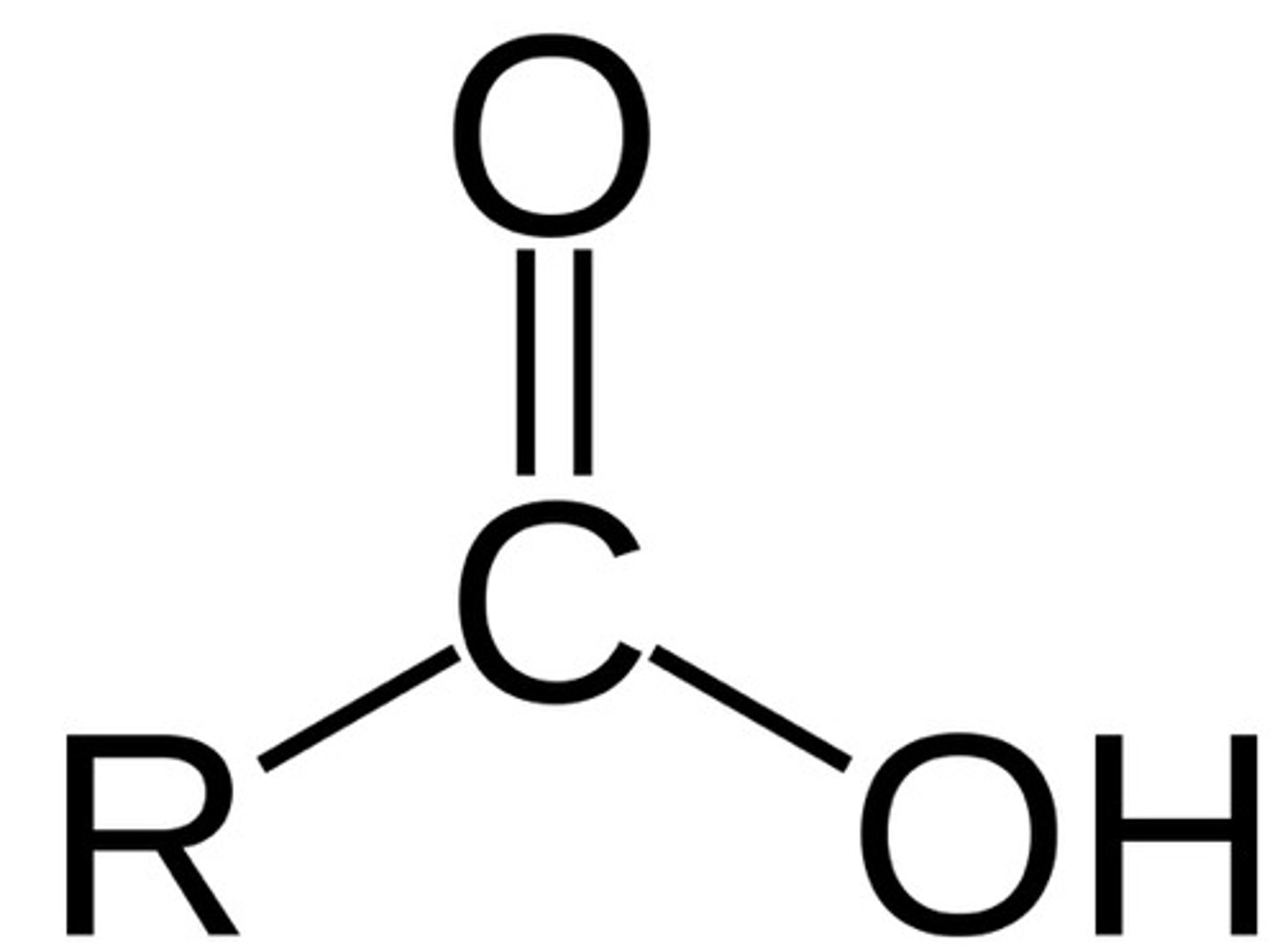
Base
Substance that accepts hydrogen ions or releases hydroxide ions (OH⁻)

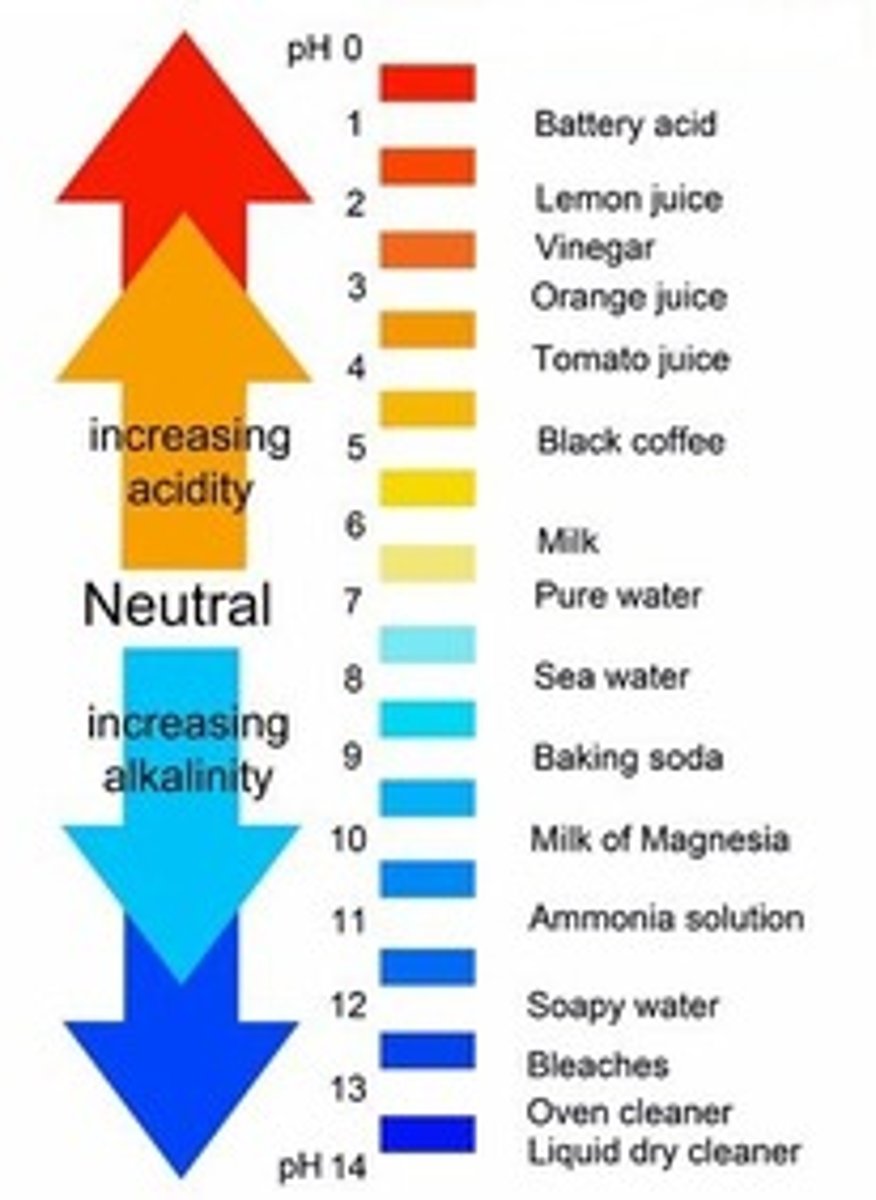
pH scale range
0 to 14
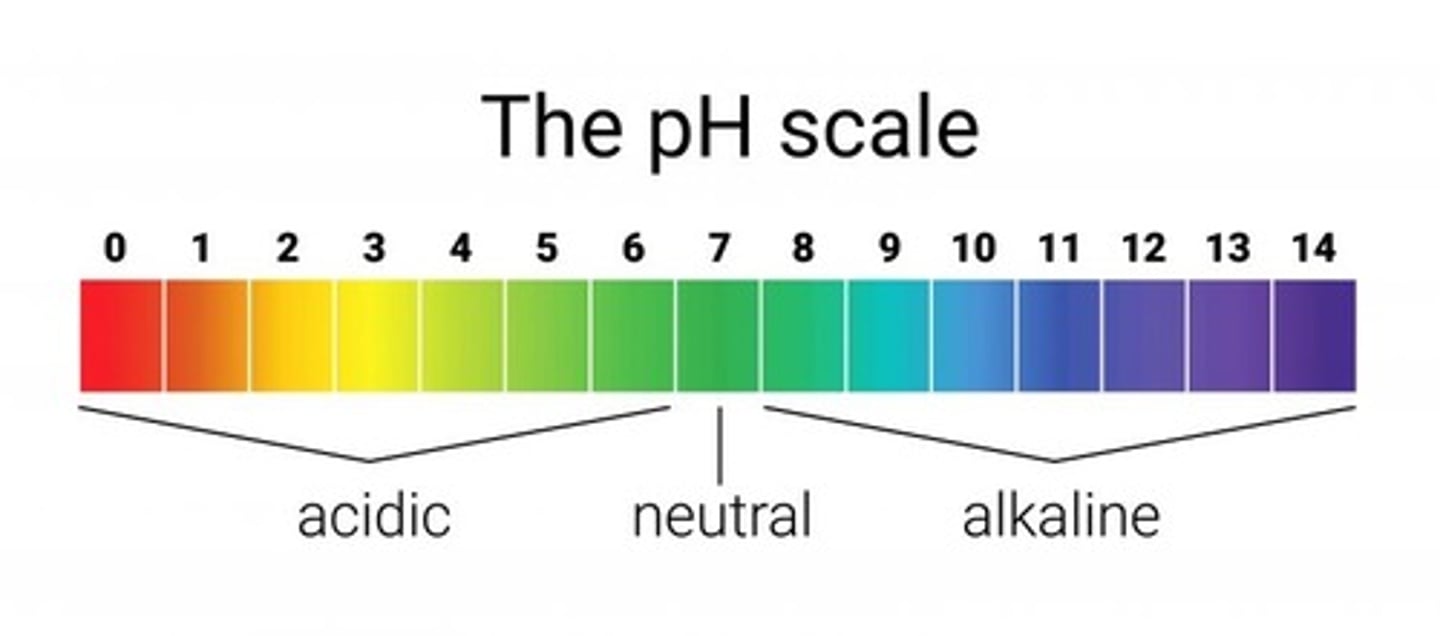
Neutral pH
7

pH less than 7
Acidic
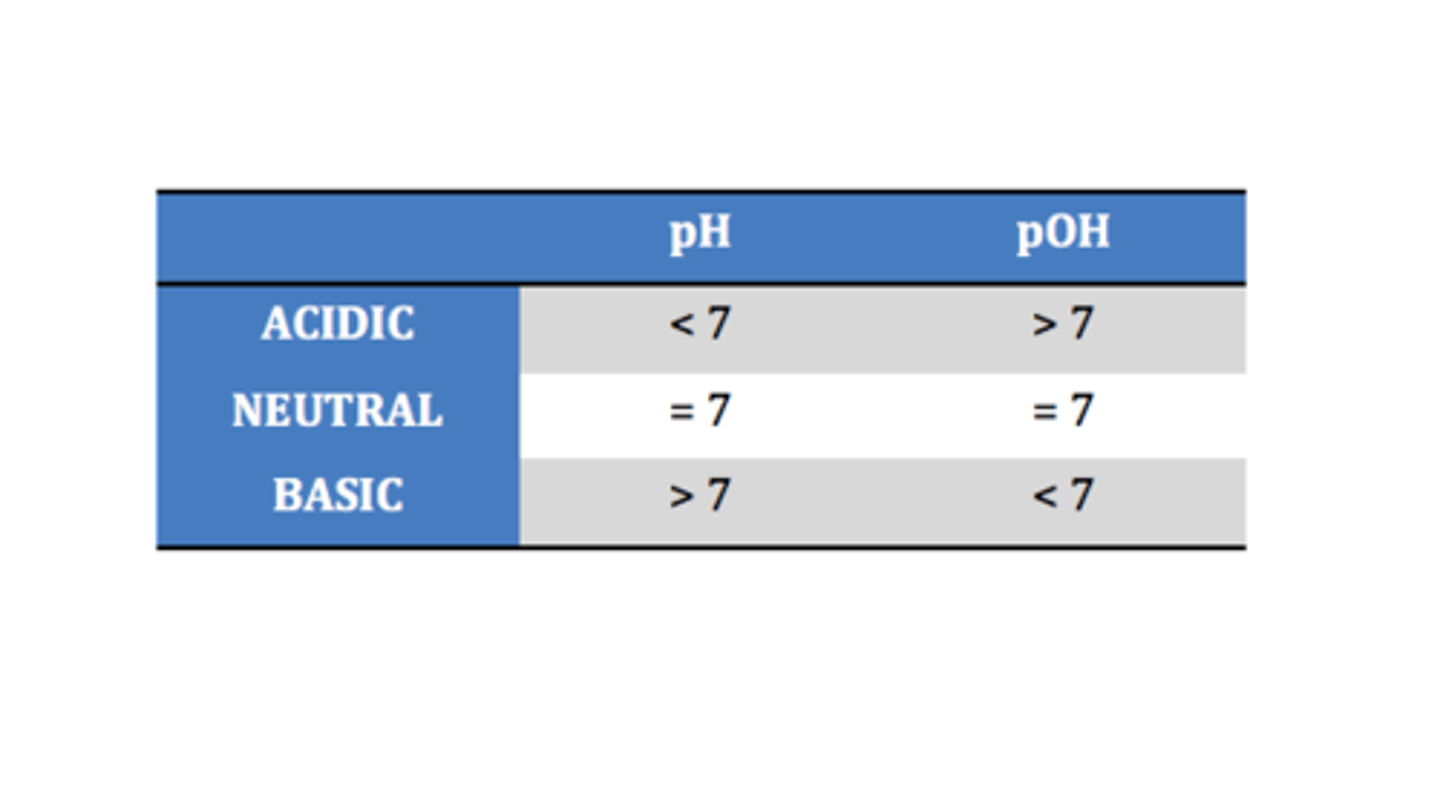
pH greater than 7
Basic (alkaline)
Strong acid
Releases many hydrogen ions
Weak acid
Releases few hydrogen ions
Strong base
Releases many hydroxide ions
Weak base
Releases few hydroxide ions
Acids taste
Sour
Bases feel
Slippery

Hydrogen ion (H⁺)
Determines acidity
Hydroxide ion (OH⁻)
Determines basicity
pH scale is
Logarithmic (each unit = 10× change)
Lower pH means
More acidic
Higher pH means
More basic
Neutralization reaction
Acid + base → water + salt
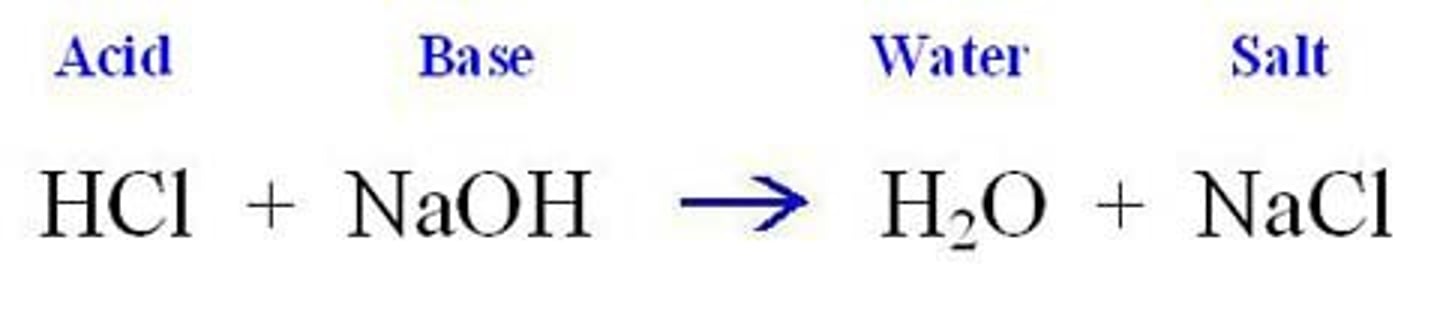
Example of an acid
Hydrochloric acid (HCl)
Example of a base
Baking soda or soap

Buffer
Substance that resists changes in pH
Why buffers are important in the body
Maintain stable blood pH
Normal blood pH range
About 7.35-7.45

Antacids function by
Neutralizing stomach acid

Strong vs concentrated
Not the same thing
Which pH value is most acidic?
1
Which pH value is most basic?
14