Everything except stuff taught by AW and AB and the tutorial questions
1/497
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
498 Terms
What is valency?
Atoms want a complete outer shell of electrons
For example, carbon has 4 electrons in the outer shell, so for a full outer shell, it needs 4 extra electrons. This is done by forming covalent bonds with other atoms and sharing the bond electrons. The valency of carbon is 4.
Valency of an atom = The number of bonds needed to complete the shell
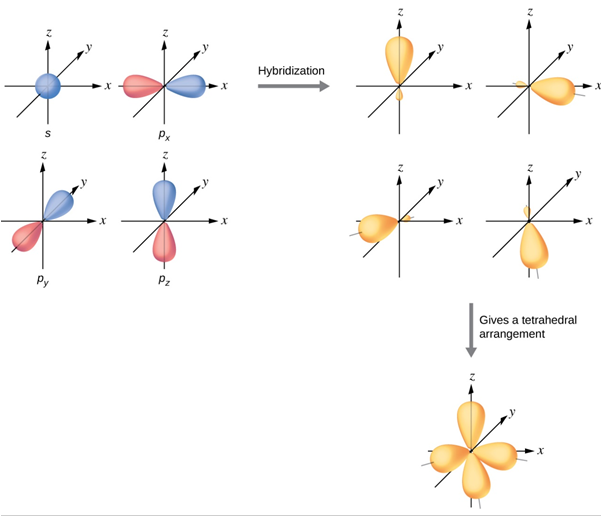
sp3
Tetrahedral
109.5
All bonds are sigma
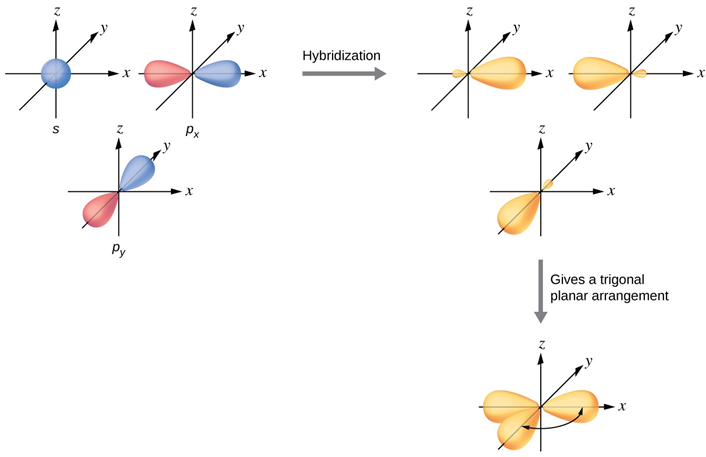
sp2
Trigonal planar
120
Bonds are single to the hydrogens, and a double bonds to carbons (sigma and pi combination)
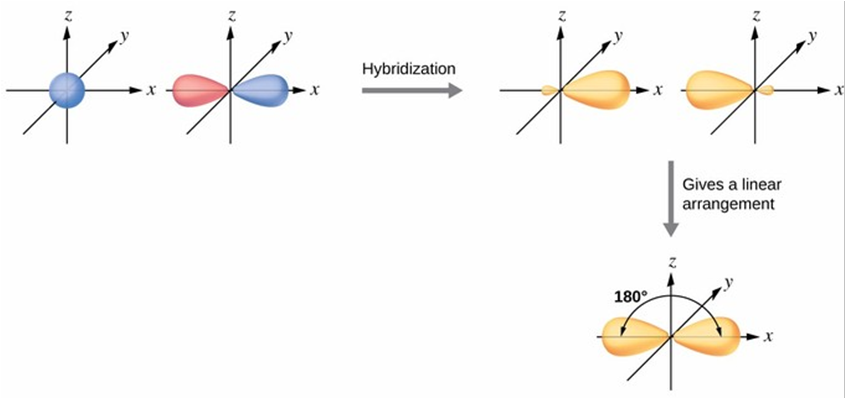
sp
Linear
180
Sigma bonds to the hydrogens and 2 pi bonds between the carbons along with a sigma bond, making a triple bond
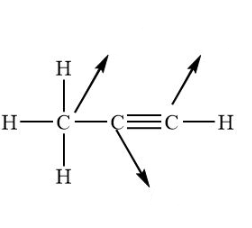
What’s the hybridisation in these molecules?
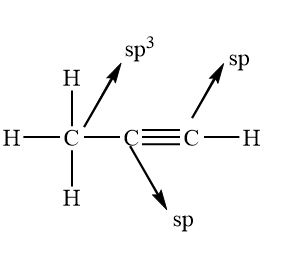
Is NH3 sp3?
Yes
Sigma bonds
Strongest type of covalent chemical bond
Forms when atomic orbitals overlap in a head on arrangement (like two 2 s orbitals, one s orbital and one p orbital)
σ
How does sigma bonding affect the shape and flexibility of drug molecules?
Sigma bonds = single bond from orbital overlap means free rotation
How does pi bonding affect the shape and flexibility of drug molecules?
Multiple bond from overlap of p-orbitals means fixed orientation
Double bonds in structures means there is…
restricted rotation
Isotopes
Atoms of the same element with the same number of protons (atomic number) but different number of neutrons (different atomic mass)
What is the kinetic isotope effect?
The change in the rate of a chemical reaction when an atom in the reactants is replaced by one of its isotopes.
What are the uses of the kinetic isotope effect?
Determining which bonds are broken in the RDS
Studying enzyme catalysed reactions
Investigating reaction transition states and bond strength differences
Name the three types of radiation
Alpha, beta, gamma
What is the exponential decay law?
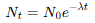
What is the Activity-time relationship?
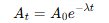
What is the link between activity and number of nuclei?
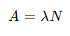
What’s the half life equation for nuclei?
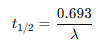
1 bq =
1 bq = 1 disintegration per second
1 Ci =
1 Ci = 3.7 × 1010 Bq

18F decays to 18O, with a half life of 109.7 minutes. What is the decay constant for 18F?
Remember to convert units if it has to be in seconds or something.

What’s the relationship between the decay constant and the half life?
The decay constant (λ) and the half-life (t₁/₂) are inversely related.
When one increases, the other decreases.
Do atoms want a have complete outer valence shell?
Yes
Atoms with nearly empty or nearly complete shells tend to ionise easily to form salts. Is there energy gain in salt formation?
Yes, the energy gain from the electrostatic interaction of the charged species.
This is an example of salt formation - ionic bonding
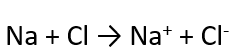
What is salt formation also known as?
Ionic bonding
Example of Ionic Bonding
NaCl
What is a covalent bond?
A chemical bond that involves the sharing of pairs of electrons between atoms
Example of Covalent Bonding
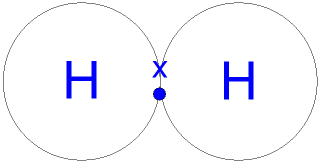
H2
The 1s orbitals of both atoms merge into a single bond orbital that contains both electrons
What is the bond dissociation energy?
When two atoms bond, their electrons and nuclei arrange into a lower-energy state, releasing energy.
The bond dissociation energy is the energy required to break this bond and separate the atoms.
It is a measure of bond strength:
Higher bond dissociation energy = stronger bond.
For a pair of atoms, greater orbital overlap → stronger bond → higher bond dissociation energy.
Name 2 types of chemical covalent bonds:
Sigma (σ) bonds
Pi (π) bonds
Are sigma (σ) bonds strong?
Strongest type of covalent chemical bond
How do sigma (σ) bonds form?
Forms when atomic orbitals overlap in head on arrangement
Sigma (σ) bond in H2
The 1s atomic orbitals overlapping to form the new sigma (σ) bonds, also known as a single bond
How do pi (π) bonds form?
Formed from overlap of two orbital lobes on one atom with two orbital lobes on another – in a lateral sense (side to side).
What do pi (π) bonds form with?
An existing sigma bond - thus they are double or triple bonds
Why does NH3 have bond angles of 107° instead of 109.5° (tetrahedral)
The lone pair exerts a slightly greater repulsion than the sigma bonds, compressing the bond angle from 109.5° to 107°
Why is the bond angle for H2O 104.5°?
There are 2 lone pairs on the O
What is a hydrogen bond?
A partially electrostatic attraction between a H which is bound to a more electronegative atom such as N, O, or F, and another adjacent atom bearing lone pair of electrons
Most electronegative atoms
N, O, F
Example of Hydrogen Bonding
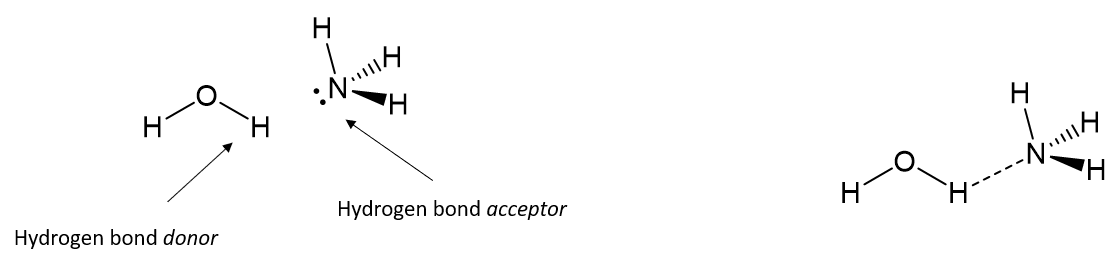
How are hydrogen bonds typically described?
Hydrogen bonds are typically described as an electrostatic dipole-dipole interaction. The δ+ of the proton is attracted to the lone pair of electrons
Hydrogen bonds are generally regarded as primarily electrostatic, however they have some covalent nature. When we say hydrogen bonds have some covalent nature, it means what?
A hydrogen bond is not purely electrostatic (attraction between charges).
There is partial sharing of electrons between the hydrogen atom and the electronegative atom it is hydrogen-bonded to (e.g. O, N, or F).
How do hydrogen bonds have a “covalent nature”?
It has direction (not like ionic bonding)
Produces bond distances that are shorter than would be expected from the sum of Van der Waals radii
In hydrogen bonds, the more electronegative the donor…
….the more covalent character is observed
Strength of Hydrogen Bonds
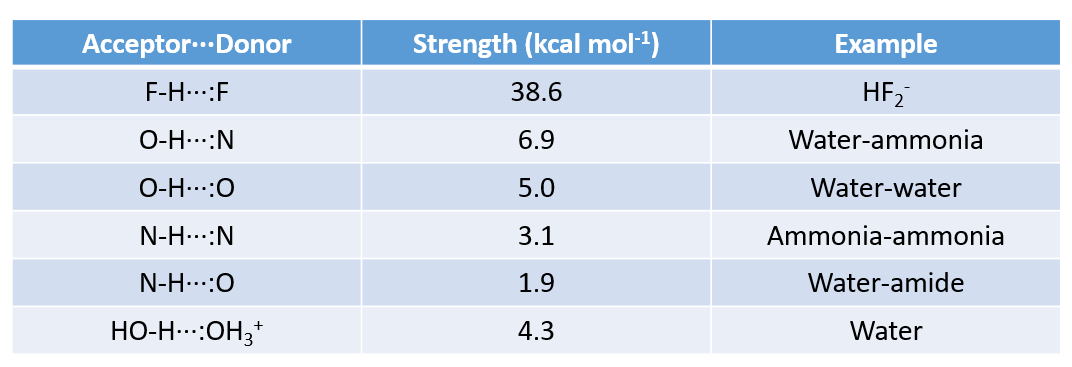
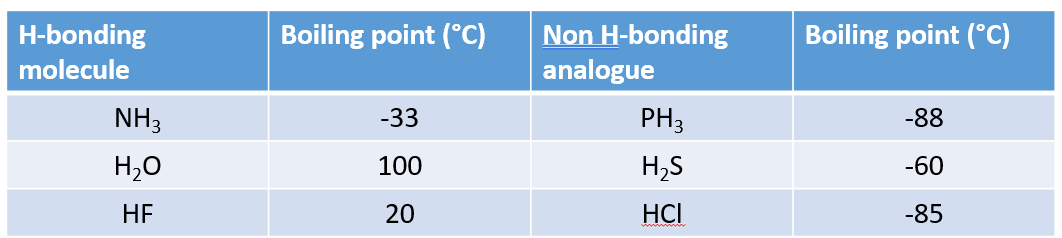
What does hydrogen bonding do in small molecules?
Increases the melting point
Increases the boiling point
Increases the solubility
Increases the viscosity
What are Van der Waal forces?
Weak intermolecular attractions that act between all atoms and molecules.
aka London forces
Weakest type of intermolecular force
Present in all molecules
Strength increases with:
More electrons / larger atoms
Greater surface area
Where do Van der Waal forces arise from?
Electrically neutral molecules may exhibit permanent electric dipoles
A permanent dipole molecule and a non permanent dipole molecule
Molecules with no permanent dipoles
1) Van der Waals forces
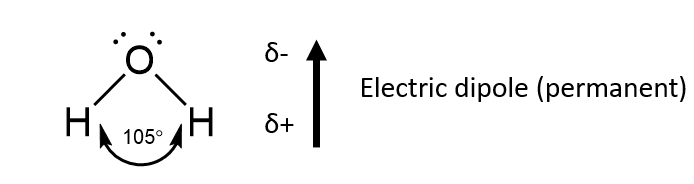
Some electrically neutral molecules may exhibit permanent electric dipoles.
For instance consider water – a bent molecule with a bond angle of around 105°.
The oxygen atom is always somewhat negatively charged and the hydrogen atoms (side) somewhat positive
These dipoles have a tendency to align, and this results in a net attractive force.
Note – this is a lot smaller than any H-bonding interaction present (e.g. in water).
2) Van der Waals forces
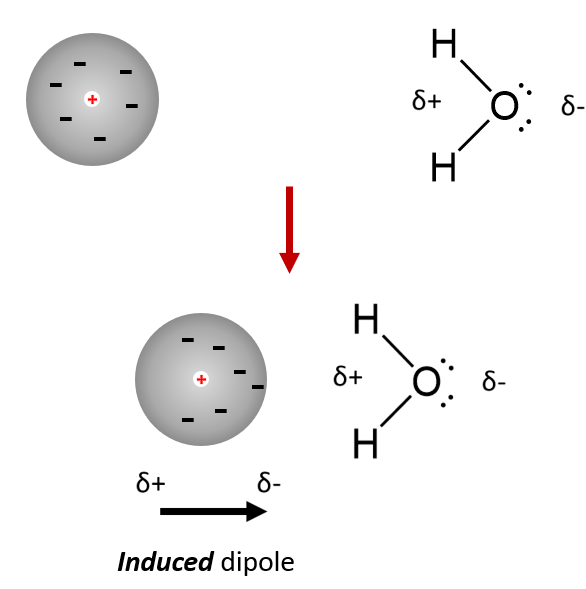
Molecules with a permanent dipole may temporarily distort the electric charge in a nearby molecule (polar or non-polar).
The extra attraction is between the permanent dipole and the ‘induced’ dipole on the nearby molecule.
3) Van der Waals forces
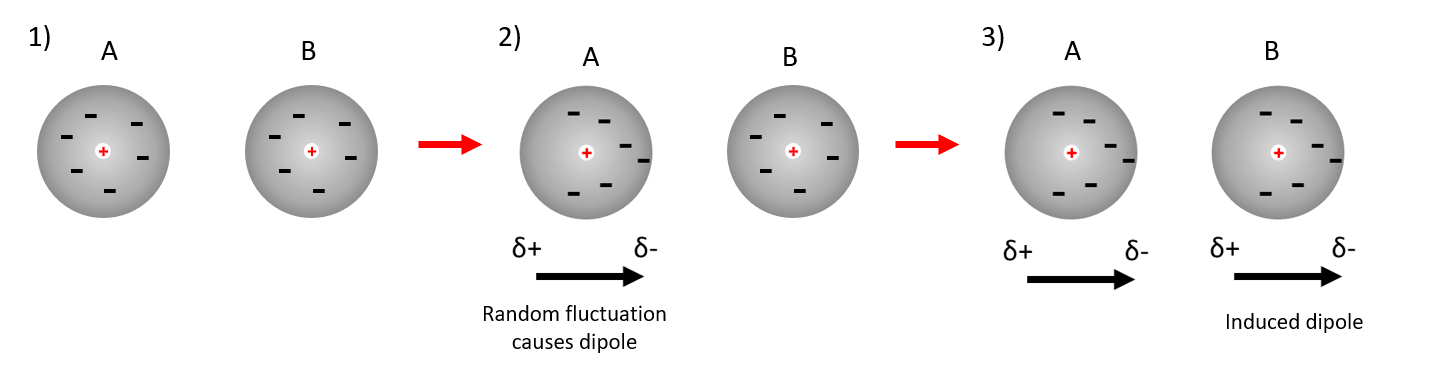
Thirdly in molecules with no permanent dipole, temporarily, dipoles result from the random electron motion within the atoms.
At any given time the centre of positive charge arising from the nucleus and the centre of negative charge arising from the electrons are unlikely to coincide.
This leads to instantaneous, but short lived dipoles, even though over time the average polarisation is zero.
The resulting instantaneous dipoles are too short lived to align with other molecules to give an attractive force, however they can induce polarization in adjacent molecules.
These specific interactions (forces) are known as London forces, or dispersion forces.
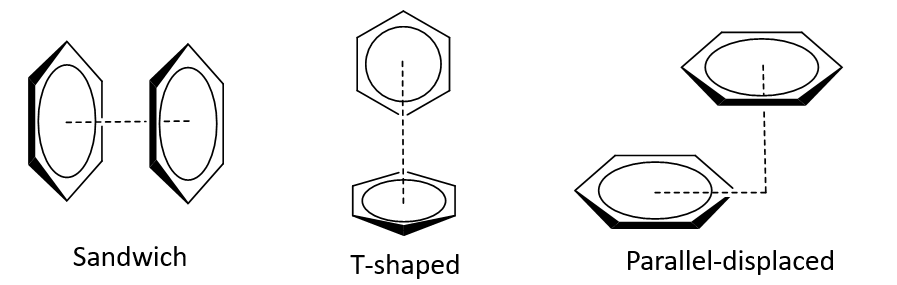
What is pi stacking (π- π interactions)?
Pi stacking refers to attractive, noncovalent interactions between aromatic rings (which contain pi (π) bonds).
What are the 3 recognised arrangements of pi stacking (π- π interactions)?
Hydrophobic interactions
Water features a relatively strong network of hydrogen bonds.
In solution this network is dynamic (constantly changing).
Non-polar molecules, such as hydrocarbon chains, cannot form hydrogen bonds, and thus attempts to mix result in breaking up of the h-bond network.
What is an amphiphile?
Molecules with both hydrophobic and hydrophilic domains
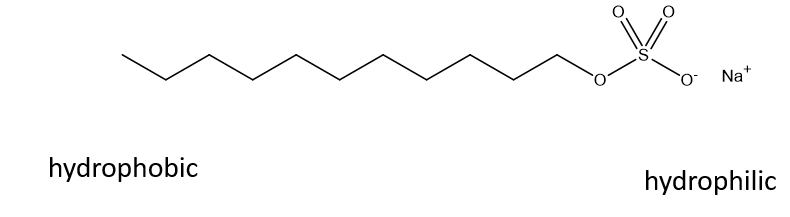
What will amphiphilic molecules form in water?
Micelles
Hydrophilic heads outside
Hydrophobic tails inside
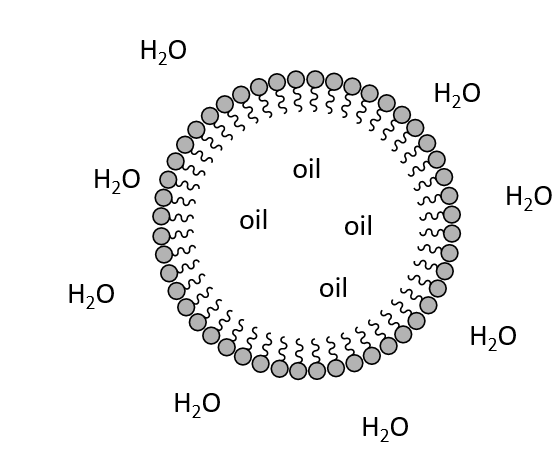
Why are micelles biologically important?
Proteins obviously contain residues (amino acids)
Amino acids can have strongly hydrophobic elements, like glycine, alanine, valine, etc
When the protein folds into its 3D shape, it is common to have the hydrophobic core compose of these residues
Charged and polar residues can interact with the surrounding water molecules
Minimizing the number of hydrophobic solvation sphere is the principle driving force behind the folding process
Primary structure of Protein
Refers to the sequence of amino acids in the polypeptide chain. The chain is held together by peptide bonds (formed from the acid group of one and the amine group of the other).
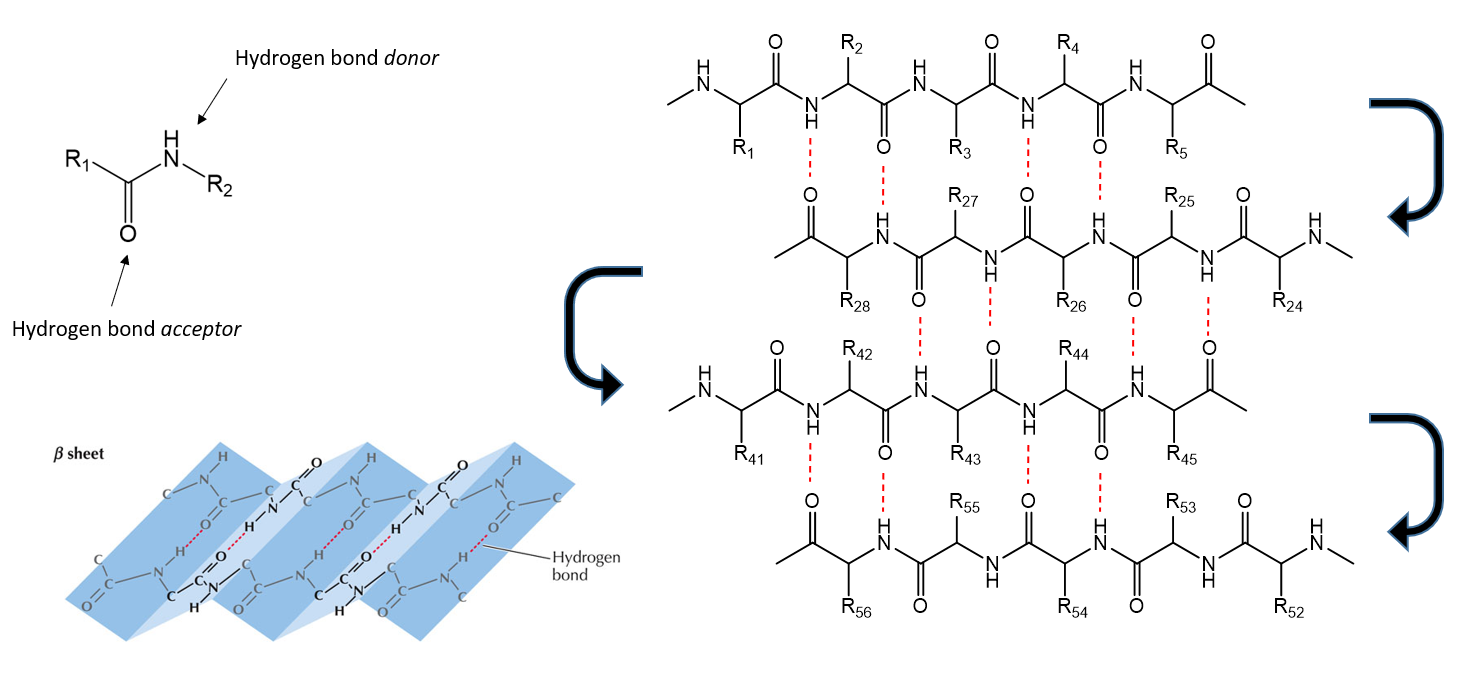
Secondary structure of Protein
Refers to highly regular sub-structures on the polypeptide backbone chain. Two main types are the α-helix and the β-sheet, and they are defined by patterns of hydrogen bonds between the main-chain peptide groups.
Tertiary structure of Protein
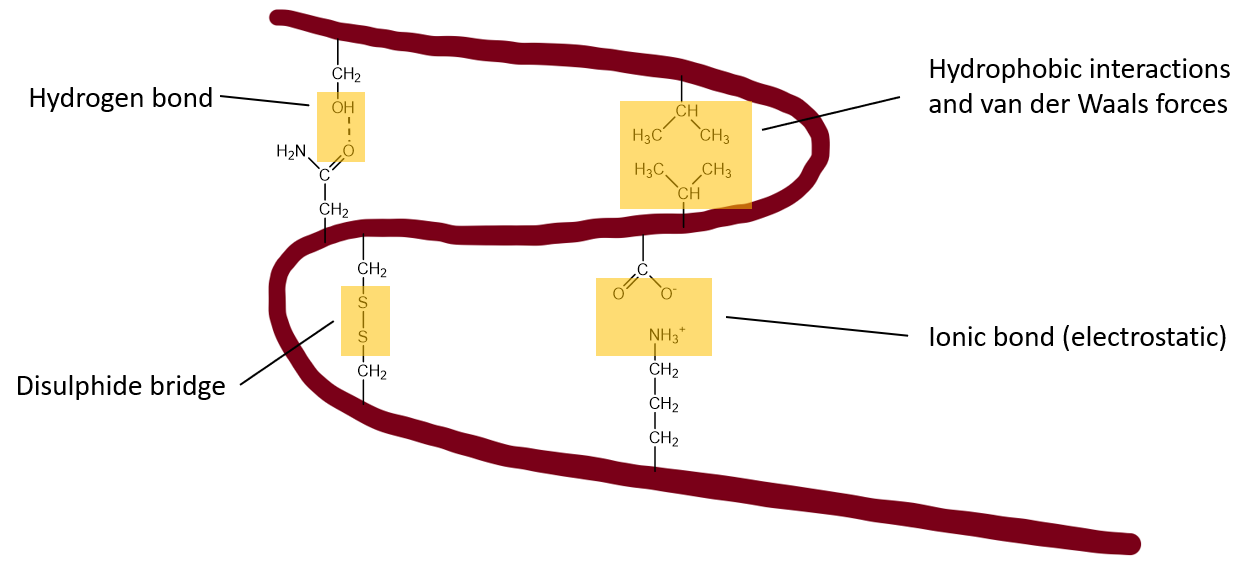
Refers to the overall three dimensional shape of the protein molecule. The α-helices and the β-sheets are folded into a compact globular structure. This is primarily driven by the non-specific hydrophobic interactions (the burial of the hydrophobic residues in the core, away from water). However further stability is also introduced by some specific tertiary interactions such as salt bridges, hydrogen bonds, the tight packing of side chains (van der Waals) and disulphide bonds.
Quaternary structure of Protein
Refers to assemblies of two or more individual polypeptide chains into one single functional unit (stabilized in a similar way to the tertiary structure).
The primary structure of a protein is reported starting at which end?
Amino terminal (N) end
Protein secondary structure - β-sheets
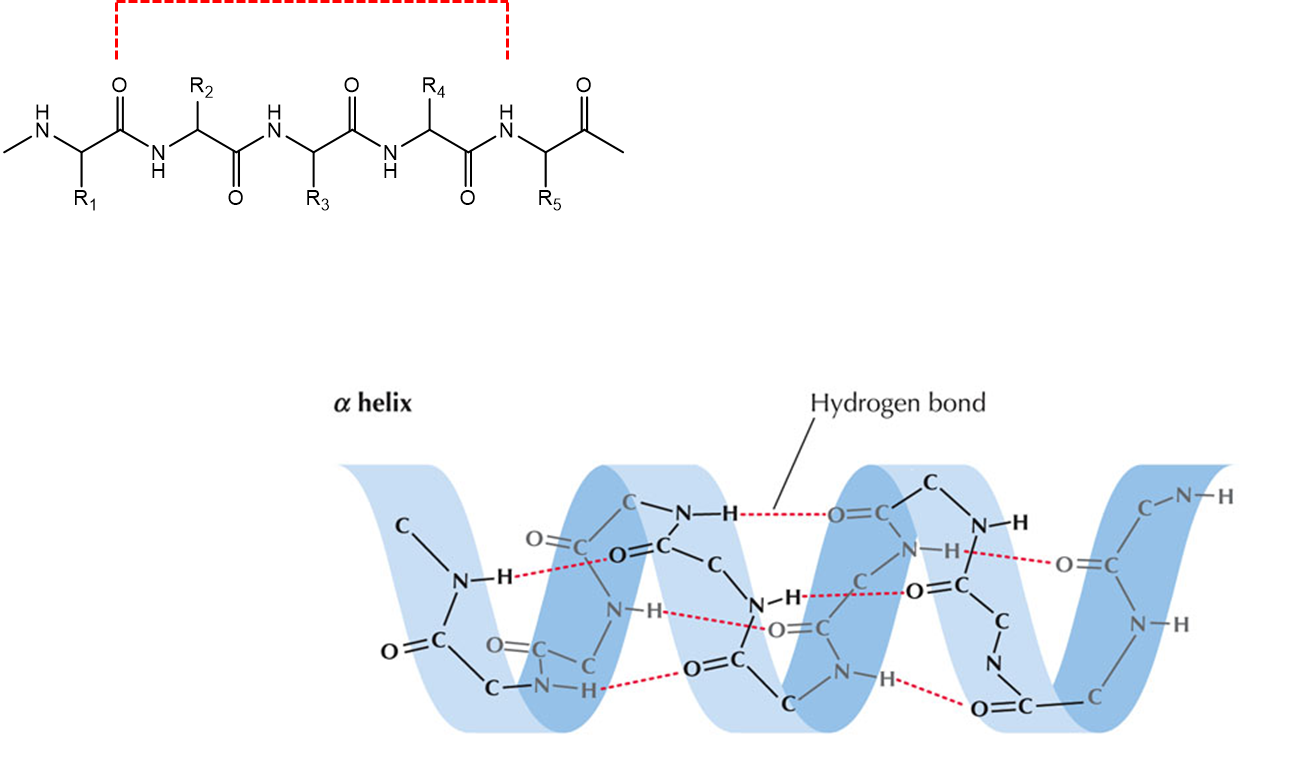
Protein secondary structure – α-helix
The carbonyl of residue n interacts with the amide proton of residue n+4.
Tertiary and Quaternary structure
The hydrophobic effect is the primary driving force in protein folding – the hydrophobic residues are forced into core of the forming globular structure, while the hydrophilic residues remain at the surface.
The gain in energy resulting from van der Waals forces in the hydrophobic regions is a big contributor to the stability of the folded protein.
Additional factors are π-stacking, salt bridges and disulphide bonds.
Definition of Electronegativity
A chemical property that describes the tendency of an atom to attract a shared pair of electrons (or electron density) towards itself
Electronegativity is determined by what?
Nuclear charge (more protons = more pull)
Number and location of electrons in atomic shells
How does electronegativity change across a periodic table?
Increases from left to right of the periodic table, and from bottom to top
F is the most electronegative
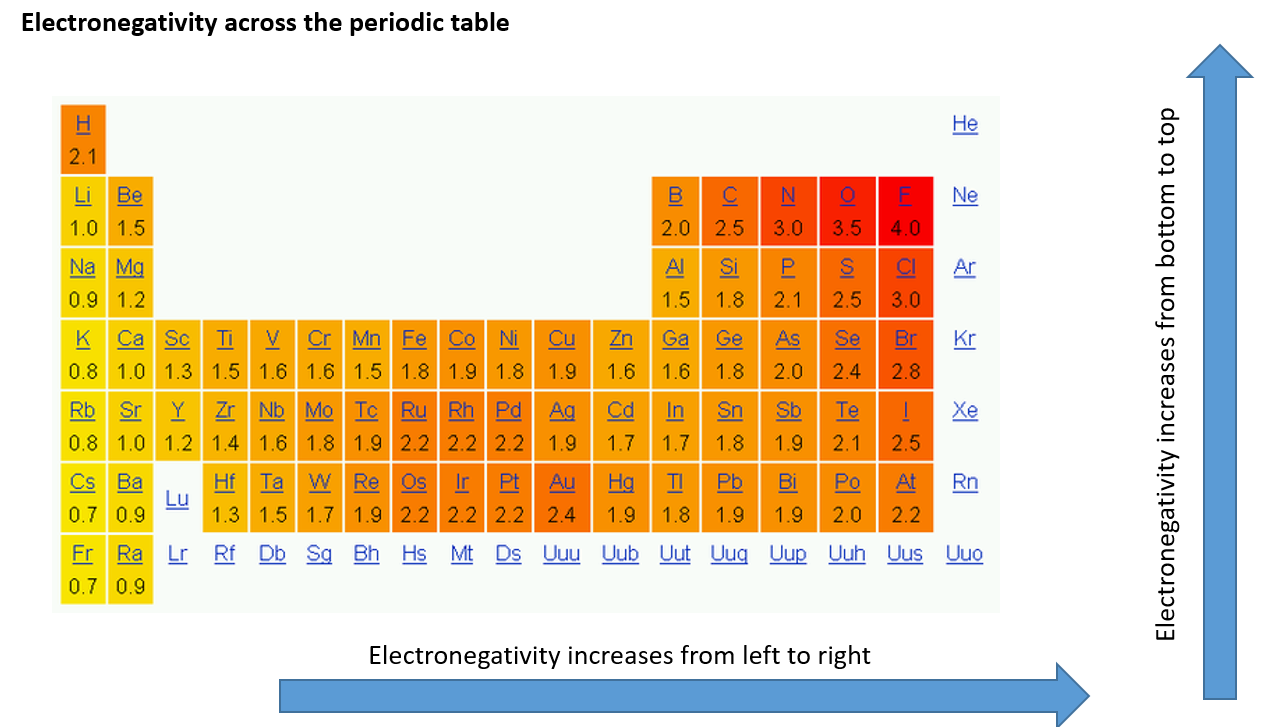
What explains the reduction in electronegativity when you descend a group?
There is extra shielding of the shells of electrons
What effect does electronegativity have on charge distribution of a molecule?
When there are difference in electronegativity, atoms will become slightly positive (δ+) and slightly negative (δ-)
This leads to induction and inductive effect
The stronger the electronegativity….
….the stronger the inductive effect
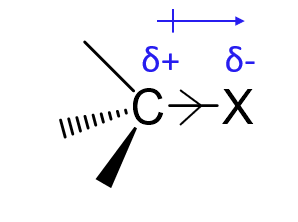
What is the inductive effect?
The permanent shifting of electron density along σ-bonds due to electronegativity differences.
What is a -I group?
An electron-withdrawing group that pulls electron density away through σ-bonds
X =
Br
Cl
NO2
OH
SH
SR
NH2
NHR
NR2
CN
COOH
CHO
C(O)R
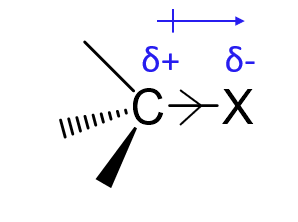
What is a +I group?
An electron-donating group that pushes electron density through σ-bonds (e.g. alkyl groups, metals).
X =
R (alkyl or aryl)
Metals (e.g. Li, Mg)
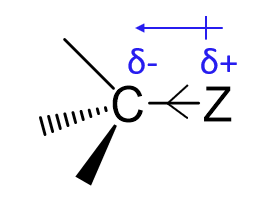
How does distance affect the inductive effect?
The inductive effect decreases rapidly as distance from the substituent increases.
What does pKa measure?
Acid strength.
Lower pKa = stronger acid = more willing to donate H⁺.
How do electron-withdrawing groups affect pKa of acids?
They stabilise the conjugate base → lower the pKa → increase acidity.
How do electron-donating groups affect pKa of acids?
They destabilise the conjugate base → raise the pKa → decrease acidity.
What is resonance?
The delocalisation of electrons over several atoms, represented by multiple canonical forms.
Why does resonance stabilise molecules/ions?
Because charge is spread over a larger area, lowering energy and increasing stability.
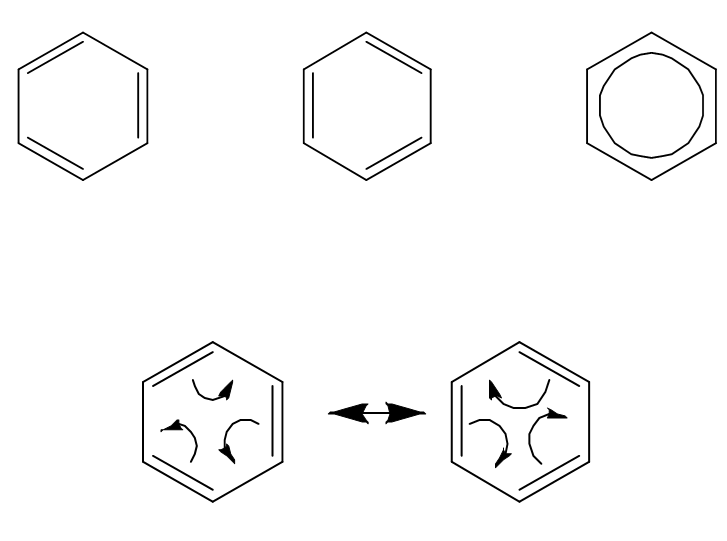
Why are benzene bonds all the same length?
Because π electrons are delocalised over the ring (true structure is a resonance hybrid).
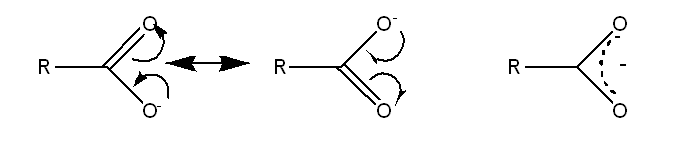
Carboxylate resonance forms
Why are resonance forms useful?
Resonance forms are useful for showing electron movement and reactivity, even though none is fully correct.
Curly arrows are used to show the movement of bond electrons between resonance forms.
Definition of the Mesomeric effect
Electron donation or withdrawal through resonance involving π systems and lone pairs.
Double bond adjacent to a lone pair of electrons
+M
When electron density is pushed into the π-bond (orbital).
-M
Where a π-orbital overlaps with an adjacent p-orbital that is low in electron density.
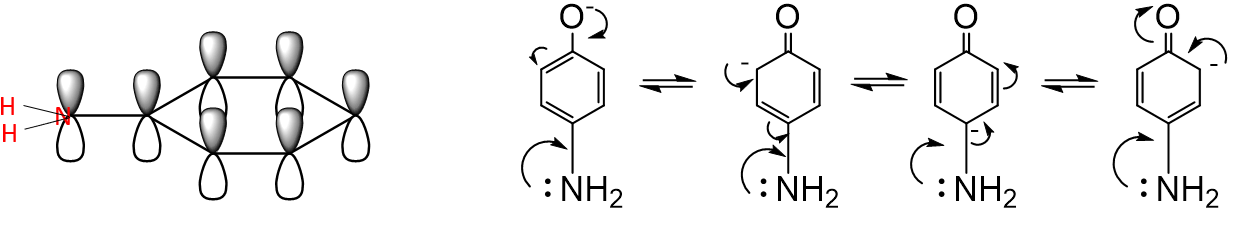
How do +M groups affect phenol acidity?
They destabilise the phenoxide ion → increase pKa → make phenol less acidic.
How do –M groups affect phenol acidity?
They stabilise the phenoxide ion → decrease pKa → make phenol more acidic.
What does pKa mean for bases?
It refers to the protonated form of the base; higher pKa = stronger base.
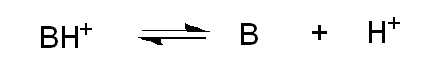
What happens when pH = pKa for a base?
About 50% of the base is protonated.
Why are alkyl amines more basic than ammonia?
Alkyl groups donate electron density (+I), making nitrogen more able to accept H⁺.

Why is phenylamine much less basic than cyclohexylamine?
The lone pair in phenylamine is delocalised into the aromatic ring, reducing availability to bind H⁺.
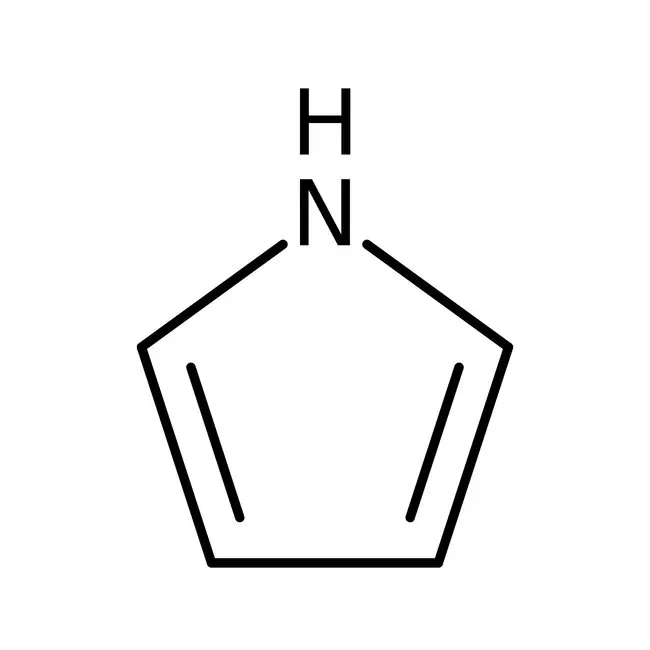
Why is pyrrole very weakly basic?
Its lone pair is part of the aromatic system and cannot accept a proton.
Why are amides extremely weak bases?
The lone pair on nitrogen is delocalised into the carbonyl group by resonance.
Why are carbonyl groups polar?
Oxygen is more electronegative than carbon, giving O δ⁻ and C δ⁺.
How do substituents affect carbonyl reactivity?
Electron-withdrawing groups increase δ⁺ on carbon → more reactive
Electron-donating groups decrease δ⁺ → less reactive
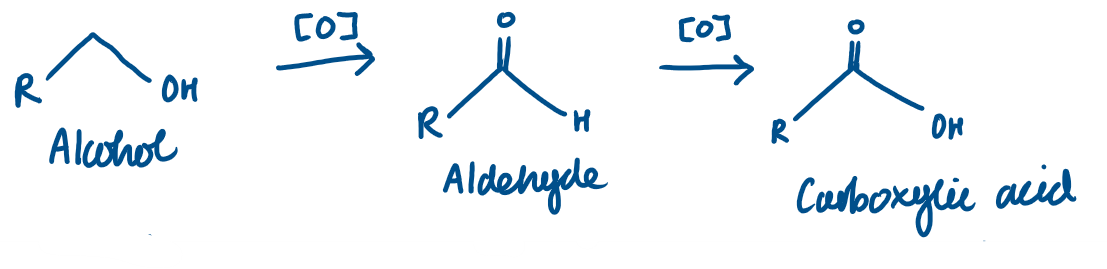
Alcohol → Aldehyde → Carboxylic acid
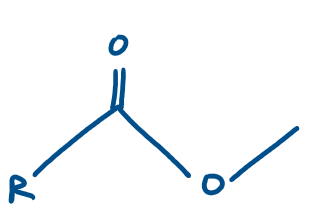
Ester
Ether
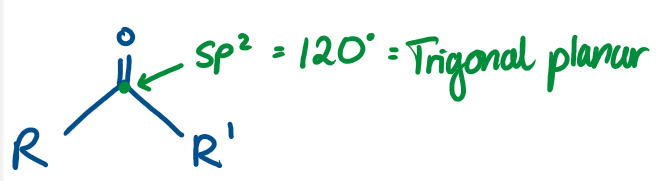
Ketone