chapter 3 bonding
1/33
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
34 Terms
ionic bonding
Ionic bonding involves electrostatic attraction between oppositely charged ions in a lattice. The formulas of compound ions, eg sulfate, hydroxide, nitrate, carbonate and ammonium.
simple covalent bond
A single covalent bond contains a shared pair of electrons.
dative covalent bond
bond contains a shared pair of electrons with both electrons supplied by one atom
metallic bonding
Metallic bonding involves attraction between delocalised electrons and positive ions arranged in a lattice.
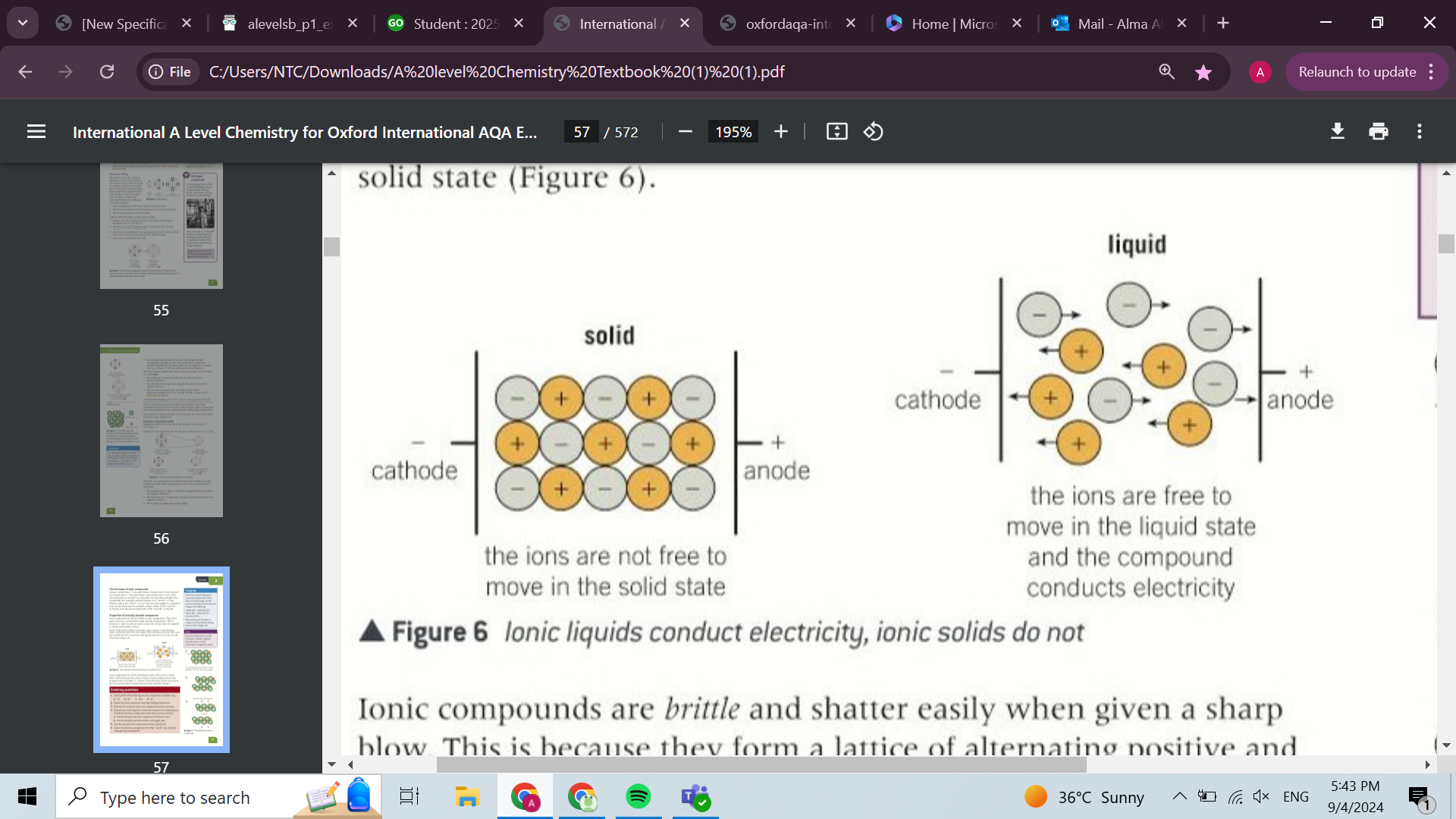
physical properties of ionic bonding (4)
brittle because once the lattice structure is disturbed ions with the same charge line up and repel each other
solids at room temperature
high melting points due to their large structures that require lots of energy to break the ionic bonds
conduct electricity when molten or dissolved because the ions are free to move and carry current
physical properties of metallic bonding
conduct electricity because they have a ‘sea’ of delocalised electrons
conduct heat due to delocalised elctrons and the ions being closely packed
malleable
ductile
high melting/boiling point
strength of a metallic bond depends on
the charge on the ion: greater charge→ more delocalised electrons → stronger electrostatic forces of attraction
the size of the ion: smaller the ion is the closer the electrons are to the nucleus
physical properties of gaint covalent/macromolecular bonding
high melting and boining points
solid at room temperature
do not conduct heat
do not conduct electricity
physical properties of simple covalent structures/ molecules
low melting
boiling points,
low solubility
are not good conductors of electricity.
diamond structure
tetrahedral with 1 carbon bonded to 4 other carbon atoms
graphite structure
hexagonal sheets with weak van der waal’s forces
1 carbon bonded to 3 other carbons
ice structure
regular lattice where the molecules become arranged into a 3D network where each water molecule is connected to four other water molecules by hydrogen bonds. As a result of this regular lattice structure, the water molecules in solid ice are further apart than the molecules in liquid water. The structure is more open in ice which makes it less dense than water.
iodine structure
diatomic molecule
Iodine has a simple molecular structure. Iodine atoms are bonded in pairs by covalent bonds, so the formula is I2. In the solid state, the weak intermolecular forces between the iodine molecules hold them together in a 3D lattice structure. This is a simple molecular lattice.
magnesium structure
tightly packed lattice and exists as a large macromolecular structure (ie. a large interconnected lattice NOT molecules) with strong metallic bonds between the Magnesium ions and the sea of delocalised electrons.
sodium choride
a crystalline lattice structure, where each sodium ion is surrounded by six chloride ions and each chloride ion is surrounded by six sodium ions.
bonding pairs and how they react
Bonding pairs and lone (non-bonding) pairs of electrons as charge clouds that repel each other. Pairs of electrons in the outer shell of atoms arrange themselves as far apart as possible to minimise repulsion.
stronger bond pairs?
Lone pair–lone pair repulsion> lone pair bond pair repulsion> bond pair–bond pair repulsion.
electro negativity
the power of an atom to attract a pair of electrons toawrds itself in a covalent bond.
trend going down group 1
Decreases because as you go down the group, the size of the ion increases, there is a weaker electrostatic force of attraction between the protons and delocalised electrons, thus requires less energy to break the forces.
types of intermolecular forces
Van der Waals (London dispersion forces)
Dipole - dipole
Hydrogen bonding
shapes of molecules and their bond angles
Linear – 180
Trigonal planar – 120
Tetrahedral 109.5
Trigonal bipyramid – 90 & 120
Octahedral - 90
bond polarity in covalent bonds
elements with different electronegativities will make a compound with an unsymmetrical electron distribution. this makes a polar covalent bond creating a permanent dipole
why do some molecules with polar bonds do not have a permanent dipole
The presence of symmetrical bonds in a molecule means that the delta charges cancel each other out.
permanent dipole dipole
polar→ non polar strongest inetrmolecular force the attraction between two permanent dipoles
induced dipole dipole aka van der waal’s
non-polar and non-polar occurs exist between all atoms or molecules when the electrons are more on one side so a temporary slight charge occurs this depends on:
higher mass= stronger van der waal’s
more electrons: stronger forces
second strongest intermolecular force
hydrogen bonding
only occurs when nitrogen ocygen and fluorine react with hydrogen. this is the strongest type of intermolecular force due to it being highly polarised
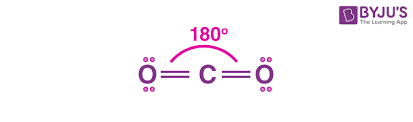
linear molecule
bonding pair:2
lone pair:0
bond angle:180 degrees
trigonal planar
bonding pair:2
lone pair:0
bond angle:120
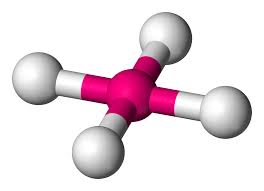
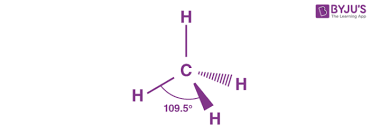
tetrahedral
bonding pair:4
lone pair:0
bond angle:109.5
trigonal bipyramid
bonding pair:5
lone pair:0
bond angle: 90 and 120
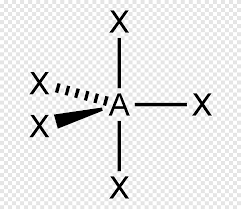
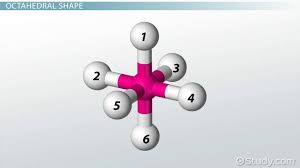
octahedral
bonding pair:6
lone pair:0
bond angle:90
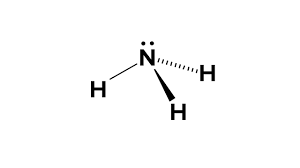
trigonal pyramid
bonding pair:3
lone pair:1
bond angle:107 degrees
bent
bonding pair:2
lone pair:2
bond angle:109 degrees
square planar
bonding pair:4
lone pair:2
bond angle:90